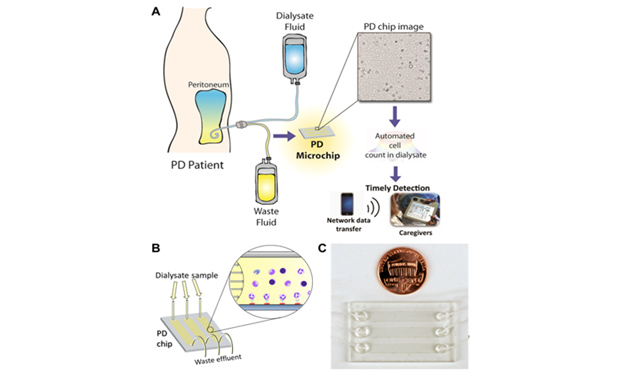
Above, Figure 1 – Monitoring infection in peritoneal dialysis (PD) patients using a microchip. (A) Neutrophils, if present in waste dialysate, are captured on a PD microchip. Next, a shadow image of neutrophils is analyzed using automated software. (B) PD dialysate is injected into microchannels. Neutrophils are selectively captured on the channel surface. Lensless imaging of the shadow of captured neutrophils in microchannels using a charge-coupled device (CCD). (C) An image of the PD microfluidic chip.
End-stage renal disease is a growing problem in the US and worldwide
In 2012, Medicare spent $32.9 billion on the treatment of, and medications for patients with end stage renal disease (ESRD), who typically need dialysis [1]. It is estimated that more than half a million patients in the US will be treated with dialysis in 2020 [2]. Peritoneal dialysis (PD) and hemodialysis (HD) are the two methods to treat kidney failure. Each year, Medicare spends ~$87K for each HD patient and ~$66K on each PD patient. It was also reported that 1.3% of Medicare recipients had ESRD and the related costs of care for this population accounted for 6.3% of the total Medicare budget. Clearly, treatment of ESRD results in a significant economic burden. On the other hand, PD is a relatively inexpensive option and is a home-based therapy compared to the HD, offering higher patient satisfaction in terms of mobility and convenience. However, a major barrier to broad adoption of PD, is the risk of infection of the peritoneum, i.e., peritonitis.
Early detection of peritonitis could prevent several dreaded complications of peritoneal dialysis
To detect peritonitis at the POC, DxNOW Inc. was established in 2013 to enable early treatment of infection, decrease fatalities and complications using a microchip coupled with lensless imaging (Figure 1, above). We have previously developed a lensless imaging technology for capturing and counting blood cells such as CD4 T lymphocytes [4], and tested them for AIDS patients in Tanzania to monitor their treatment and CD4 cell levels [5]. Similarly, we then tailored this strategy to capture and count neutrophils from PD effluent in the event of infection. Therefore, we developed specific surface chemistry approaches to selectively capture neutrophils by immobilizing anti-CD66b antibody on the microchannel surface. Once the neutrophils are captured on the surface, we leverage the capability of lensless imaging technology to visualize the neutrophils using an inexpensive charge-coupled device (CCD) and to rapidly report the number of neutrophils on the lensless image using an automated cell-recognition algorithm. This technology can help patients and caregivers to take timely measures, and hence, potentially monitor patients self-administering PD especially those who already have a standing prescription.
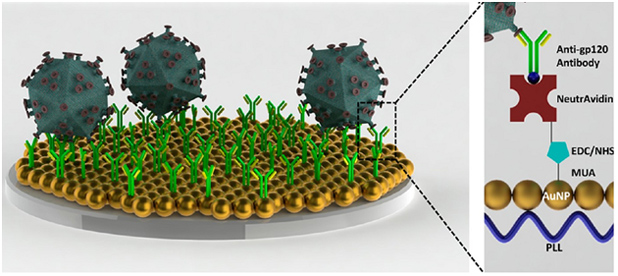
Recently, we have developed a sensitive nanoplasmonic platform technology, which employs localized surface plasmon resonance (LSPR) strategy for detection of biotargets (e.g., cells, biomarkers, and viruses) from biological samples (Figure 2) [6]. In principle, this nanoplasmonic technology monitors the collective oscillation of conduction electrons from the metal nanoparticles induced as a result of the capture of a specific biotarget, leading to the change in wavelength and extinction intensity parameters [7]. Due to the generation of “hot spots” on the nanoplasmonic surface between adjacent gold nanoparticles, the signal of spectral color is enhanced, allowing highly sensitive detection. As a proof-of concept, we facilitated this platform technology to detect and quantify HIV viruses from unprocessed whole blood and HIV-infected whole blood patient samples as a model system, with high sensitivity down to ~100 copies/mL. Further, this versatile technology can detect neutrophils and lymphocytes as well as bacteria in a quantitative manner in microfluidic chips, creating an ideal platform for POC testing. For early detection of peritonitis, we demonstrated rapid, accurate and repeatable detection of biotargets including white blood cells (i.e., neutrophils) and bacteria (i.e., Escherichia coli (E. coli))[8] with a wide dynamic range. Thus, early detection of peritonitis by this platform would improve patient care and allow more patients to access peritoneal dialysis at home, improving the patients’ quality of life, enabling independent living, and reducing the overall cost of caring for these patients.
In conclusion, lensless imaging and nanoplasmonic platform technologies have shown great potential for achieving on-site detection of peritonitis in patients on PD. Commercialization of these microchips can significantly increase the adoption of PD for the treatment of ESRD patients and reduce healthcare cost, thus enabling their use at home.
Acknowledgments
We would like to acknowledge National Institute of Health (NIH) RO1AI093282, NIH RO1AI081534, NIH U54EB15408, NIH R21AI087107, NIH R01AI076442, NIH U54AI057159, NIH/NIAID R56AI091889, NIH/NIAID R01AI079085, Brigham and Women’s Hospital (BWH) BRIght Future Prize, and Epilepsy Foundation Shark Tank Prize.
Competing Financial Interests
Dr. U. Demirci is a founder of, and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions, and (ii) Koek Biotech, a company that is developing microfluidic IVF technologies for clinical solutions.
References
- S. R. D. System, “USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases,” 2012.
- A. J. Collins, R. N. Foley, D. T. Gilbertson, and S.-C. Chen, “The State of Chronic Kidney Disease, ESRD, and Morbidity and Mortality in the First Year of Dialysis,” Clinical Journal of the American Society of Nephrology, vol. 4, pp. S5-S11, December 1, 2009 2009.
- J.-B. Nousbaum, J.-F. Cadranel, P. Nahon, E. N. Khac, R. Moreau, T. Thevenot, et al., “Diagnostic accuracy of the Multistix 8 SG reagent strip in diagnosis of spontaneous bacterial peritonitis,” Hepatology (Baltimore, Md ), vol. 45, pp. 1275-81, 2007.
- S. Moon, H. O. Keles, A. Ozcan, A. Khademhosseini, E. Haeggstrom, D. Kuritzkes, et al., “Integrating microfluidics and lensless imaging for point-of-care testing,” Biosens Bioelectron, vol. 24, pp. 3208-14, Jul 15 2009.
- S. Moon, U. A. Gurkan, J. Blander, W. W. Fawzi, S. Aboud, F. Mugusi, et al., “Enumeration of CD4+ T-cells using a portable microchip count platform in Tanzanian HIV-infected patients,” PLoS One, vol. 6, p. e21409, 2011.
- F. Inci, O. Tokel, S. Wang, U. A. Gurkan, S. Tasoglu, D. R. Kuritzkes, et al., “Nanoplasmonic Quantitative Detection of Intact Viruses from Unprocessed Whole Blood,” ACS Nano, May 20 2013.
- O. Tokel, F. Inci, and U. Demirci, “Advances in plasmonic technologies for point of care applications,” Chem Rev, vol. 114, pp. 5728-52, Jun 11 2014.
- S. Wang, F. Inci, T. L. Chaunzwa, A. Ramanujam, A. Vasudevan, S. Subramanian, et al., “Portable microfluidic chip for detection of Escherichia coli in produce and blood,” International journal of nanomedicine, vol. 7, p. 2591, 2012.