Innovative methods that are simple to run and offer real-time results provide insight on health
Ions can say a lot about a baby’s health. High levels of chloride in sweat can indicate cystic fibrosis (CF), a disorder that causes respiratory and digestive problems; abnormal sodium levels are signs of increased risk for seizure or muscle problems; and too-high or too-low potassium concentrations can cause heart arrhythmias or other muscle issues. These ions—commonly called electrolytes— are also important throughout life. For instance, too much or too little sodium can lead to confusion, seizures, and muscle weakness or cramping.
Hospital clinicians typically test for these ions using invasive or rather cumbersome methods that require post-test analysis and offer a view of a patient’s ion concentrations at one moment in time. Today’s engineers and researchers, however, are devising new measurements that are simple to run, offer real-time results, and in some cases, provide continuous monitoring. One group is even using changes in ion flow—skin electrical resistance—as possible indicators of breast cancer.
Stick-on patch for CF
To help diagnose CF, clinicians typically use a hard plastic device that straps onto the baby’s arm. Epidermal application of a chemical (pilocarpine) and mild electrical stimulation induces perspiration, the baby’s sweat collects in a coil within the device, and is then transferred to a benchtop instrument that performs the analysis and reports the concentration of chloride ions. “We were alerted by pediatricians at the Ann & Robert H. Lurie Children’s Hospital in Chicago that this process is not ideal for a number of reasons,” said John A. Rogers, Ph.D., (Figure 1) whose research group at Northwestern University in Evanston, IL, USA, had been developing skin-applied stickers for other purposes, including the measurement of sweat rate and sodium loss for athletes [1].
“The pediatricians asked if we could adapt our work to do screening for CF, because the standard device is uncomfortable (in that) it must be strapped tightly to the skin to avoid any leakage of sweat. And even so, it often fails to collect sufficient volumes of sweat to obtain an accurate reading,” Rogers said. “Besides that, the measurement approach requires an expensive desktop machine, called a chloridometer.”

Figure 1. John Rogers and his Northwestern University research group are developing skin-applied, sweat-analyzing stickers. One of their stickers is now commercialized as a sweat-monitoring device for athletes, and others are in the works for other uses, including the diagnosis of cystic fibrosis.) (Photo courtesy of Northwestern University.)
As it turned out, it was not a big leap to go from a sweat sticker for sports to one for CF, remarked Rogers, who is director of the Querrey Simpson Institute for Bioelectronics, and a professor of materials science and engineering, BME, and neurological surgery at Northwestern. They used similar microfluidic channels to collect the sweat, but because they were no longer targeting athletes who perspire heavily, they designed the CF sticker with much smaller channels. “And while it’s the same reaction chemistry for the bioanalytics in both applications, we’re doing it in much smaller reservoirs for CF and using a built-in assay that changes color by an amount determined by the concentration of chloride,” he said.
They designed the CF sticker as a soft piece of silicon elastomer that gently conforms and adheres to the surface of the baby’s skin (Figure 2). The clinician need only place a sticker on a baby’s arm, use a phone to take a picture of it once it changes color, and read the precise chloride measurement, Rogers said. “And it does so at a much lower cost, because you no longer need the chloridometer or other hospital infrastructure that goes along with it.”

Figure 2. The Northwestern CF detecting device is a flexible sticker with built-in microfluidic channels that gather sweat from the baby’s skin, route it through the channels, and via a color change, nearly instantaneously report whether the sweat has the high chloride levels that are indicative of CF. (Photo courtesy of Tyler Ray/University of Hawai‘i at Ma¯noa.)
Rogers and his group have already fully validated the sticker against the standard of care, and found it yields colorimetric assessments of chloride without any loss of accuracy or precision [2]. Researchers have now begun human testing of an upgraded version of the CF patch that delivers both pilocarpine and a minute charge via tiny gel electrodes, so the sweat-induction capabilities are also built in (Figure 3). Rogers added, “Ultimately we’re interested in translation and commercialization of this system. All of the various aspects and components have already been ‘de-risked’, all the way to high-volume manufacturing and quality control, so it looks very promising.”

Figure 3. Early patches required prior application of the chemical pilocarpine to stimulate sweat production, but the researchers have made the patch even easier to use by including the pilocarpine application in the patch (an older version of the patch shown here). Testing of the upgraded patch is under way. They are also currently collaboratively investigating the possibility of sweat stickers to detect other biomarkers, such as calcium, zinc, and iron. (Photo courtesy of Tyler Ray/University of Hawai‘i at Ma¯noa.)
In other work, the Northwestern research group is also collaboratively investigating the possibility of using sweat stickers to detect other biomarkers, such as calcium, zinc, iron, and vitamin C [3]. “We published a paper recently showing that concentrations of these nutritional biomarkers in sweat track those in blood, so you can watch time variations after you take a vitamin tablet,” Rogers said. “With these integrated, all-in-one devices, we can do multiple assessments at the same time using a single device. That represents an important future for the technology.”

Figure 4. W. Hong Yeo at Georgia Tech leads a large research team developing a variety of technologies, including a smart pacifier to monitor electrolyte concentrations in babies. Such a pacifier could take the place of blood draws. (Photo courtesy of the Yeo research group.)
Smart pacifier for electrolyte monitoring
Suggestions from the medical community were the impetus for a Georgia Institute of Technology (Georgia Tech) project that put ion-reading technology into a pacifier, but this time its purpose was to measure sodium and potassium levels in a baby’s saliva, according to W. Hong Yeo, director of the Institute for Electronics and Nanotechnology (IEN) Center for Human-Centric Interfaces and Engineering, and associate professor and Woodruff Faculty Fellow of mechanical and BME at Georgia Tech (Figure 4). The suggestions followed talks he had given in Atlanta, Georgia, and in South Korea, where he discussed his group’s noninvasive health-monitoring systems, including a mouth guard that quantifies sodium intake [4]. After both talks, clinicians approached him about whether his group could adapt its systems for infants.
He was intrigued, so after obtaining funding from the IEN Center and South Korean government, Yeo and researchers from that country began collaborating on technology that could turn a typical commercial pacifier into a baby-friendly electrolyte detector. “Electrolyte levels are very important metrics that clinicians have to look at on a daily basis, particularly for sick babies, and the way they do that today is through a periodic blood draw,” Yeo said. “That is problematic for infants because they don’t have developed blood vessels for easy and repeated blood draws, and they also get bruised very easily. In addition, blood draws can only be done a maximum of three times a day and have to go to a lab for analysis, so monitoring can neither be continuous nor real-time.”
Their answer was to develop a microfluidic channel system, ion-selective sensors, flexible circuitry, and wireless bluetooth capability that detects ions in saliva and fits into a pacifier. “We used one of the commercially available pacifiers, but this would work with any pacifier,” Yeo said. “Everything is embedded inside, so nothing has direct contact with the baby and it is completely safe.” They also generated an app that sends a continuous data stream to a clinician’s phone or tablet, which can be accessed in real time or as a recording, he noted.
The group faced numerous challenges while developing the smart pacifier, including devising microfluidic channels that could efficiently pump saliva, which is a viscous fluid, and then transport sufficient quantities to the sensor area for quick measurement (Figure 5). “We spent a lot of time changing materials and surface properties in a way that we can get nice accumulation and concentration of saliva,” he said. Initial testing with babies in a neonatal intensive care unit at Yonsei University Hospital in South Korea showed that the technology worked well to measure both sodium and potassium levels [5] and further studies are under way there to continue evaluating its feasibility and performance.
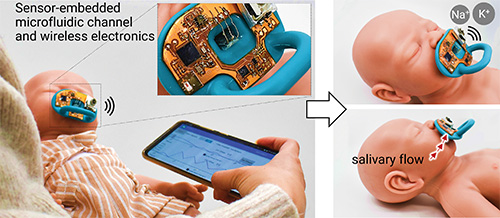
Figure 5. Yeo’s group developed a microfluidic channel system, ion-selective sensors, flexible circuitry, and wireless Bluetooth capability that detects ions in saliva and fits into any commercially available pacifier. This prototype displays the electronic components. The researchers also developed an app to deliver continuous data to a clinician’s phone or tablet either in real time, or as a recording. (Photo courtesy of the Yeo research group.)
Back at the Georgia Tech, Yeo’s group is designing a machine-learning-based algorithm to identify and automatically alert clinicians when a baby experiences potentially problematic changes in sodium or potassium levels. “In terms of sensors, we also hope to go beyond sodium and potassium, and detect other ions, too,” he said. He is co-founder of a new spinoff company called WisHealth, also in Atlanta, which is currently commercializing a sticker-type technology that works like a stethoscope but continuously monitors heart and lung sounds in pediatric patients. In addition, he noted, WisHealth will likely soon begin commercialization of the pacifier.
Yeo credits much of the group’s success and energy to its large size and diversity—three engineers, nine postdocs, and about two dozen graduate students with backgrounds ranging from mechanical, BME, electrical, and chemical engineering to biology and computer science—as well as to their drive to make a difference. “We do problem-driven research, we work with many collaborators to identify and tackle existing issues, and we are focusing on biomedical issues and helping people who need our help right now, today,” he commented. “It’s challenging, but very meaningful.”
Skin ionic detector for cancer
The work on a new detection method for cancer uses a completely different approach that centers not on specific ion concentrations, but on overall ionic changes in the body affecting the flow of electrical current.
The idea behind the work was to find a complement to mammograms, said Benjamin Sanchez-Terrones, Ph.D., assistant professor of electrical and computer engineering at the University of Utah, Salt Lake City, UT, USA (Figure 6). Mammograms have a couple of major drawbacks: they use radiation, which limits screening frequency typically to no more than once a year and no earlier than age 40, and it often is unable to find cancer in women who have dense breast tissue, he said. This means women with dense breast tissue—nearly half the female population—receive mammogram results that are inconclusive, and young women don’t get mammograms even though cancer can strike before age 40.

Figure 6. Benjamin Sanchez-Terrones, Ph.D., assistant professor of electrical and computer engineering at the University of Utah, is developing a noninvasive, radiation-free technology that detects changes skin resistance to electrical current flow to help detect breast cancer. It could have benefit for women who are too young to receive standard mammography screening, and for those who have dense breast tissue and receive inconclusive results from mammograms. (Photo courtesy of Dan Hixson/University of Utah College of Engineering.)
For their approach, Sanchez-Terrones and his research group concentrated on the body’s response to cancer. “Cancer triggers the immune system, and as a result of that, there is a cascade of changes to the interstitial, lymphatic fluids that alters how electrical current flows through your body, so we decided to develop a way to detect this change in current,” he summarized.
They started with a straightforward setup: a pair of electrodes. The patient holds one in her hand, and the clinician holds a second electrode-containing probe that generates entirely painless electrical current. “The current goes from the patient’s hand to whatever body location the operator is touching, so the operator begins by touching an arm as a reference point, and then compares that reading with several taken from the breast,” he explained (Figure 7). “The measurements only take a few seconds. From there, we have a machine-learning algorithm that has been trained on data gathered from women who have breast cancer, and it determines if a patient may have breast cancer or not.”
The University of Utah group has been working with the company IONIQ Sciences of Salt Lake City, UT, USA (Sanchez-Terrones is on the company’s scientific advisory board), to develop the technology and conduct testing. Their preliminary clinical study of 48 patients showed that the technology correctly determined the presence or absence of cancer 70.7% and 75.1% of the time, respectively [6]. In nondense tissue, mammogram is more effective at diagnosing cancer, he acknowledged, “but our method is noninvasive, it doesn’t involve radiation, it works in women who have dense breast tissue, and it can be used on women of any age, so it is an attractive adjunctive technology.”
The study also measured a subset of cancer patients before surgery and again six months afterward, and observed changes pre- to post-treatment. “This indicates that the technology could have potential to detect longitudinal changes and therefore could be used to follow therapeutic treatment in these women, and if necessary, adjust it on the fly,” Sanchez-Terrones said, noting that it might also provide useful information for drug developers.
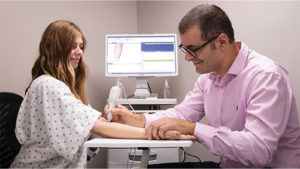
Figure 7. In the University of Utah’s approach, the patient (demonstrated by Natasha Andreasen of IONIQ) holds an electrode while the medical professional (Sanchez-Terrones) measures current with a probe that delivers a low-voltage electrical pulse. Differences in current between a reference point (the arm in this case) and sites in the breast are a potential sign of cancer. Collaborating company IONIQ Sciences is developing the technology for commercialization. (Photo courtesy of Dan Hixson/University of Utah College of Engineering.)
IONIQ Sciences is pursuing U.S. Food and Drug Administration approval for the technology, and is also exploring its use for identifying other types of cancer. As that proceeds, Sanchez-Terrones and his research group are collaborating with a radiologist at the University of Utah to refine the technology and continue patient studies to confirm the technology’s effectiveness.
Sanchez-Terrones reiterated that his group is not trying to replace mammography. “Mammography is here to stay, and it’s a good starting point, but our technology opens the door to allowing more people to be screened and to catching breast cancer earlier, and that’s something women and their doctors care about.” <
References
- Epicore Biosystems. Gx Sweat Patch Provides Hydration Biomarker Analytics and Recovery Insights. Accessed: May 12, 2022. [Online]. Available: https://www.epicorebiosystems.com/gx-sweat-patch/
- T. R. Ray et al., “Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management,” Sci. Transl. Med., vol. 13, no. 587, p. eabd8109, Mar. 2021, doi: 10.1126/scitranslmed.abd8109.
- J. Kim et al., “A skin-interfaced, miniaturized microfluidic analysis and delivery system for colorimetric measurements of nutrients in sweat and supply of vitamins through the skin,” Adv. Sci., vol. 9, no. 2, Jan. 2022, Art. no. 2103331, doi: 10.1002/advs.202103331.
- Y. Lee et al., “Wireless, intraoral hybrid
electronics for real-time quantification of sodium intake toward hypertension management,” Proc.
Nat. Acad. Sci. USA, vol. 115, no. 21, pp. 5377–5382, May 2018, doi: 10.1073/pnas.1719573115. - H.-R. Lim et al., “Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes,” Biosensors Bioelectron., vol. 210, Aug. 2022, Art. no. 114329, doi: 10.1016/j.bios.2022.114329.
- N. Andreasen et al., “Skin electrical resistance as a diagnostic and therapeutic biomarker of breast cancer measuring lymphatic regions,” IEEE Access, vol. 9, pp. 152322–152332, Oct. 2021, doi: 10.1109/access.2021.3123569.



