In the field of wearable biomedical sensors, the convergence revolution is more than a fanciful, utopian view of the way innovation should be done. Medical-grade wearable sensors rely on it. Their development requires technical know-how, computing expertise, clinical input, and collaboration—a true meeting of the minds to permit the conversion of wearables from neat gadgets into practical and proficient tools that will propel health care to new heights.
“It’s the intensive projects that involve engineers, mathematicians, and other researchers teaming up with physiologists and clinicians that are really going to get us ahead. And that’s what we call the convergence revolution, where engineering, math, and physics contributions to bioscience are going to make a huge difference,” says Karl Friedl, Ph.D., retired as director of the Telemedicine and Advanced Technology Research Center for the U.S. Army Medical Research and Material Command in Frederick, Maryland, and adjunct professor of neurology at the University of California, San Francisco. He cites as an example the development of gait sensors for patients with Parkinson’s disease (see “Biosensors Promise Better Days for Parkinson’s Patients, Others”).
[accordion title=”Biosensors Promise Better Days for Parkinson’s Patients, Others”]
Parkinson’s disease is a condition that is primed for wearable sensors, says Karl Friedl of the University of California, San Francisco. Sensor-based movement diagnostics will help to remove some of the disparities between clinicians in assessing patients, he continues. These disparities, known as inter-rater variability, arise because of the subjective nature of scoring patients in standardized movement tests.
For instance, one standardized test involves the patient getting up from a chair, walking a certain distance, and coming back to the chair to sit down. Clinicians score the patient based on their observations, but the scores can vary from one clinician to the next. “If we had some kind of wearable gait-measuring device that would do this automatically and consistently, it would reduce the interrater variability for this assessment,” he maintains.
Wearable movement diagnostics will also be helpful for monitoring patients at home or for remote consultations with physicians through Skype or other means, he says. Physicians who note a concerning movement issue could then connect the patient with a neurologist. This has implications for early diagnosis and prompt treatment, Friedl says. “Through telemedicine, we can potentially identify more people who have Parkinson’s disease, connect them remotely to the limited number of neurologists who are trained in this field, and make a huge impact on patients.”
In addition, sensor-based movement diagnostics will benefit patients in their everyday lives. He points to the Basis Peak smart watch and app, developed in collaboration with the Michael J. Fox Foundation for Parkinson’s Research and Intel Corp. “It’s got everything from a simple reminder to take your medication to feedback on how the patient is doing in everyday activities, which might lead to an adjustment of a medication or a medication timing or some other treatment alteration,” Friedl explains. “The biomedical community is eagerly awaiting the results of this and related realworld monitoring efforts.”
Such unobtrusive but continuous monitoring could note subtle changes, including an emerging sleep problem or a slow decline in activity levels, that patients otherwise might not notice on their own, he notes [1]. “If you have something that’s monitoring how patients are really doing and provides some kind of record, that will be hugely helpful to their disease management.”
Wearables can make a big difference in Parkinson’s diagnosis and treatment because the disease is so amenable to measurements, especially those associated with motion and gait, he says. Not only that, he also believes that what they learn here can be extrapolated to other neurological conditions and probably to other diseases. “For instance, once we understand more about how to measure normal gait, imagine putting this in a soldier’s smart boot. We could perhaps tell that he or she is starting to drop one foot and becoming fatigued or pick up an indicator of an overuse injury that’s about to happen.”
He adds, “Beyond that, this would be helpful to healthy normal people in preventing injury.”
Reference
- J. A. Stamford, P. N. Schmidt, and K. E. Friedl, “What engineering technology could do for quality of life in Parkinson’s disease: A review of current needs and opportunities,” IEEE J. Biomed. Health Informatics, vol. 19, no. 6, pp. 1862–1872, Nov. 2015.
[/accordion]
Beyond the increasing miniaturization of hardware and the shift to wireless communication technology, flexible electronics and more powerful computing capabilities, including application-specific integrated circuits (ASICs) and microelectromechanical systems (MEMS), have enabled new work on body sensor networks (BSNs) that monitor, analyze, and make sense of body signals for the prevention, diagnosis, and treatment of health disorders. Developments in processing, such as sensor-connected nodes combined with evolving algorithms and decreasing power requirements, have also contributed. In addition, new approaches to subjective measures (pain and emotion) have opened possibilities.

“Some of these things have actually reached wide-scale deployment, and some of them are still in laboratory-controlled experiments, but they’re all in realization and at different stages of being accomplished,” says Guang-Zhong Yang, Ph.D., director and cofounder of the Hamlyn Centre for Robotic Surgery and chair of the Centre for Pervasive Sensing at Imperial College London, United Kingdom. About a decade ago, he and his research group introduced the concept of the BSN [1], where data from sensors that are worn externally or implanted into the patient are gathered, integrated, and processed, and the resulting information is transmitted wirelessly for both storage and analysis (Figure 1).
Powered by You
For Yang, body-inspired design is critical to the progression of BSNs, and power for those sensors is going to be a linchpin in that evolution (Figures 2 and 3). While sensor batteries have become longer lasting, their power still drains, and, of course, when it does, the sensors stop working. He contends that the answer lies in employing the body as the source of power. “I’m talking about power harvesting that uses the vibration or low-frequency motion of the body itself to provide continuous sensing,” he says. The development of ultralow-power mixed-signal ASICs (semiconductor chips with both analog and digital circuits) can lead to significantly reduced power requirements, so body-harvested power will be sufficient to run these devices. He remarks, “Once we can accomplish this, the entire area of biosensor monitoring will really change.”
In particular, Yang notes the need for continuously monitoring sensors that can capture episodic symptoms. In the current clinical environment, he says, a patient’s symptom may not appear when he or she is visiting the doctor, so the doctor often cannot objectively analyze it for diagnosis or treatment purposes. This leaves the physician and especially the patient feeling frustrated. “If we could provide continuous monitoring with biomedical sensors that capture what’s going on with the patient, we can then understand the context of why and under what condition a symptom is happening,” Yang says.
Several research groups are working on acquiring power from the human body, including the research group of Mehmet Yuce, Ph.D., an academic member of electrical and computer systems engineering at Monash University in Victoria, Australia, and associate editor of IEEE Sensors Journal (Figure 4). His group is designing low-power integrated circuits, or microchips, that can harvest maximum energy from different combinations of body vibration, electromagnetism, and the piezoelectric effect that the body generates as people go about their daily activities.
In one technique for this overall project, which Yuce collectively calls WE-Harvest (so named for wearable energy harvesting), he and his research group constructed a prototype device (Figure 5) that employs magnets and piezoelectric diaphragms [2]. “As the person moves—and even while just sitting, a person is still moving—the magnet inside the box moves and hits the piezo, so we get movement from the magnet as well as the piezo,” he explains. “We built the prototype to demonstrate that it works, and we are now miniaturizing it to be used with biosensors.”

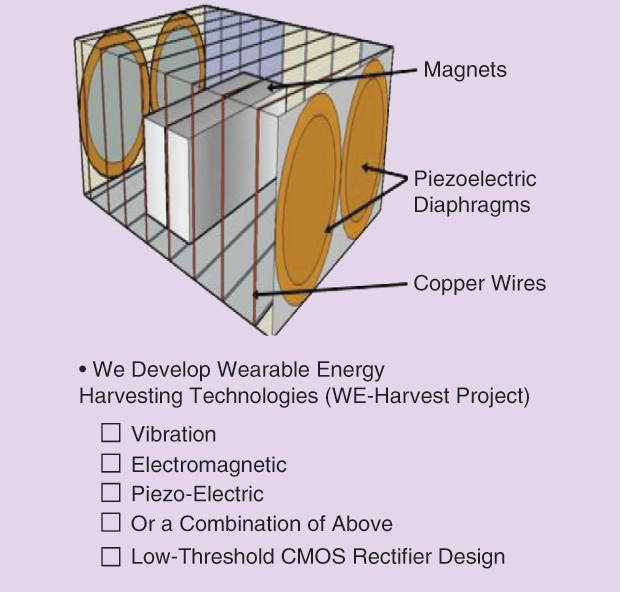
The point of WE-Harvest is to eliminate the need to change biosensor batteries by autonomously charging them, thereby providing infinite energy, he says. “This is important in many clinical applications where you need continuous data. You have to make sure the energy is there.”
While Yuce and his group developed this technology for wearable sensors, they are now also designing MEMS-based harvesters for implantable devices. Here, they use ultrasonic waves to vibrate the harvester to provide the energy. “For this, we need tiny devices, and the only way to make such small harvesters and mechanical sensors is with MEMS technology,” he explains. At the same time, they are working on a second option—a dedicated external transmitter that delivers an electromagnetic signal to the implantable device to provide the power source—and are looking into the possibility of reaping power from some of the body’s moving internal organs. Yuce comments, “We think we might be able to get some small amount of energy from the heart or other internal organs eventually, and although we haven’t tested them yet, we are developing devices to see if that’s even possible.”
If It’s Good Enough for My Body…
Power isn’t the only way BSNs can take inspiration from the body, according to Yang. As an example, he points to his group’s work on a sensor to monitor a patient’s gait. “When you talk about the gait—how you walk or run—immediately you think, ‘Let’s put a few sensors on the body, maybe on the arms and legs, to capture motion.’ The problem is that the patient doesn’t want to [wear multiple sensors]: one sensor is enough.” Instead, Yang and his research group looked at how the human body monitors gait.
“Our biological sensors are positioned in the inner ear, and that’s not by chance. This developed through years of evolution as the most economical way of sensing our motion,” Yang says. Motion-produced, high- and low-frequency waves from the lower limbs and from the trunk conduct well through the skeleton to the inner ear. Building on this biological paradigm, his research group has designed a sensor to be placed behind the ear (Figure 6). Because this site is bony, it does not attenuate the signals and yields high-fidelity information retrieval.
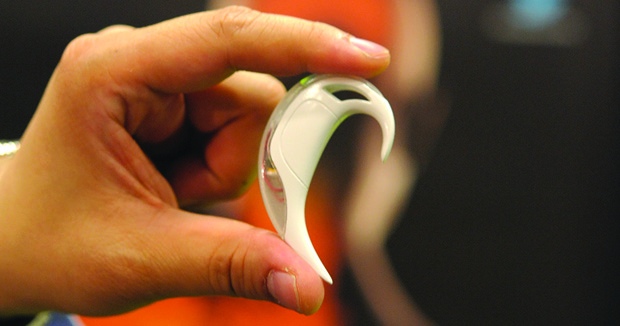
Likewise, Yang and his group are exploring biocompatible, biointegratable, and possibly even dissolvable surgical sutures or staples that are embedded with sensors to detect anastomosis failures and surgical infection, as well as injectable microsensors that have the ability to communicate [3]. “These microsensors would form a BSN that is very similar to the networks that cells or bacteria use to communicate among themselves,” he says. Such projects are very exciting to Yang. “Just looking at it from a health care economics point of view, although minimally invasive (e.g., keyhole) surgery has made significant progress over the years, if we cannot discharge patients safely, all the potential gain will be lost,” he says. New sensor networks that can monitor and assess patients for potential problems such as infection, however, would allow patients to go home quickly and safely, and thus free up bed space for those who really need it.
He adds, “These types of projects demonstrate how the body can be used as the inspiration to design sensors that can work, and that can work well.”
Routing to the Superhighway
Another critical aspect of the BSN is sending the information to the Internet (Figure 7), Yuce says. “We can put many low-power sensors on the human body to monitor things like electrocardiograph (ECG), temperature, and all of the other required physiological parameters, but then you want that to be connected to the Internet so that you can actually see the information anytime, anywhere.” He adds, “This ‘Internet of Things’ (IoT) for the health care domain is an umbrella that covers everything from wearables to implantable devices.”
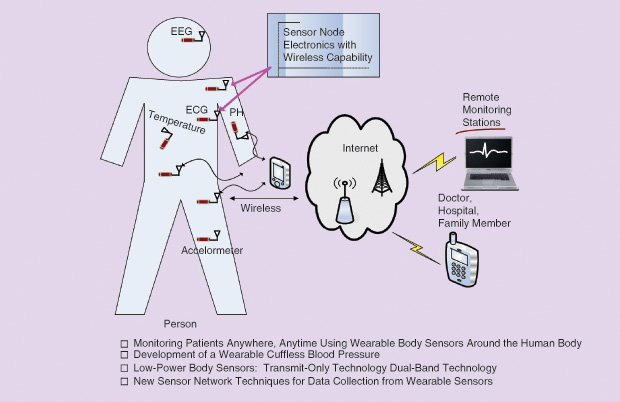
Yuce and his group have already developed a prototype wearable and cuffless blood-pressure monitor that they presented in we ever expected,” Friedl says. As one example, he notes that when researchers began studying marathon runners to protect them from heat stroke, they felt it would be a simple matter of monitoring for abnormally high core temperatures. Normally, a core temperature of 39 °C is considered dangerous, but when runners’ temperatures were monitored during a marathon race, it was found that they can hit 40 °C for two hours, and that was peak performance. “From this, researchers found that temperature alone isn’t going to tell us who’s in trouble. Instead it had to be a combination of temperature and heart rate, so it took physiologists and smart math modelers to put the two together and come up with an algorithm called the physiological strain index.”
Likewise, among soldiers participating in the grueling U.S. Army Ranger training school, the telltale indication for hypothermia was not just low core temperature but also shivering behavior, especially the cessation of shivering, according to Friedl. “This is what tells us they’re in thermoregulatory fatigue and are not responding the way they should, so it’s time to evacuate them,” he explains. Again, the hypothermia indication had to merge the expertise of researchers in more than one discipline.
Both instances demonstrate the importance of multidisciplinary interaction to the design of useful wearable and implantable sensors and BSNs, Friedl says. “A physiologist can come up with interesting observations, but they’re not useful until you model them, and a modeler can dump in data and try to come up with a neural network approach, for instance, but that ignores some of what you need to understand about the physiology,” he remarks.
The same is true of engineers, clinicians, and all of the other researchers who are contributing to this field. “There are just so many possibilities in this area where engineering meets biosciences. We need people teamed together.”
References
- G. Z. Yang, Ed. Body Sensor Networks, 2nd ed. London, U.K.: Springer, 2014.
- R. Hamid, A. Mohammadi, and M. R. Yuce, “WE-Harvest: A wearable piezoelectric-electromagnetic energy harvester,” presented at the 10th Int. Conf. Body Area Networks, Sydney, Australia, 28–30 Sept. 2015.
- J. Andreu Perez, D. R. Leff, H. M. Ip, and G. Z. Yang, “From wearable sensors to smart implants—Toward pervasive and personalised health care,” IEEE Trans. Biomed. Eng., vol. 62, no. 12, pp. 2750–2762, Dec. 2015.
- K. M. S. Thotahewa, J.-M. Redouté, and M. R. Yuce. “A lowpower wearable dual-band wireless body area network system: Development and experimental evaluation,” IEEE Trans. Microw. Theory Tech., vol. 62, no. 11, pp. 2802–2811, Nov. 2014.
- K. M. S. Thotahewa, J. Y. Khan, and M. R. Yuce. “Power efficient ultra wide band based wireless body area networks with narrowband feedback path,” IEEE Trans. Mob. Comput., vol. 13, no. 8, pp. 1829–1842, Aug. 2014.