Electricity is the currency of our nervous systems. Thinking and planning, walking and talking, eating and sleeping—all our mental and physical activities are driven by electrical signals moving through the brain. This electrical traffic ebbs and flows in consistent patterns across different brain regions, carrying information from one neuron to the next.
But this orderly scheme disintegrates during a seizure. Electrical signals run amok, producing torrents of neural activity that can cause a person to lose consciousness, shake involuntarily and disconcertingly, and even stop breathing. Although seizures can occur for different reasons, people with epilepsy are prone to seizures because a part of their brain, called a seizure focus, is too excitable and apt to ramp up into uncontrolled electrical signaling that spreads to the rest of the brain.
Medications can be used to calm the brain for many people with epilepsy, but not all. About one-third of those with epilepsy have drug-resistant seizures, leaving them vulnerable to brain damage from the unchecked electrical storms; when not incapacitated by a seizure, they remain disabled due to the risk of having one. Of those individuals with drug-resistant seizures, 10% have a seizure focus that can be surgically removed without harming the brain regions responsible for crucial functions like memory or language. “It’s obviously a very, very brutal option of last resort,” notes Dimitri Kullmann (Figure 1), a neurologist at University College London, United Kingdom. “I think of it as a brain amputation. Surely we could do better.”
Kullmann is now forging a path toward controlling seizures through chemogenetics, a method that introduces molecular control switches into the brain. Consisting of an engineered receptor that lies dormant until activated by a drug that works solely on that receptor, the technique offers a way to focally counteract the hyperexcitable seizure epicenter. One type, known as a “designer receptor exclusively activated by designer drugs” (abbreviated “DREADD”), can successfully control induced and spontaneous seizures in rodents, and Kullmann is now seeking funding for a human trial.
“We’re still at an early stage, but I think we’re very fortunate that it’s a pretty straightforward path to a clinical trial,” Kullman explains, noting that there is not that much that needs to be invented to get the technique working in humans. If all goes well, the first trial may begin in two years.
DREADDs, used for nearly ten years in rodents to reveal the neural circuits mediating a diverse array of processes (including feeding, addiction, memory, attention, and respiration), are brimming with therapeutic possibility for humans. By providing a more precise way of targeting a faulty circuit in the brain, DREADDs may avoid the side effects common with most drugs that can have widespread action throughout the brain. DREADDs may also offer a more refined but less invasive alternative to deep brain stimulation (DBS), which requires leaving a metal electrode in the brain to trigger activity in a selected brain region.
But it is early days, with researchers just beginning to contemplate the best first candidates for DREADD therapies. Translating the rodent research into humans will require working out the best way to deliver DREADDs to the human brain; finding ways to measure how well the designer receptors are taken up and engaged by the designer drug; and ensuring that the designer drug, taken by mouth, has no off-target effects. Experiments in nonhuman primates will be crucial to this translation but have only just begun, with current studies related to addiction and anxiety.
“Many brain disorders are disorders of circuitry, we think, and so DREADDs offer a much more fine-tuned way of modulating neuronal activity than the current way it’s done,” explains Bryan Roth (Figure 2) from the University of North Carolina, Chapel Hill, who first introduced DREADDs [1]. “So I think, ultimately, something like this technology will eventually be used in humans.”

On-Demand Remote Control
Roth first developed DREADDs out of curiosity, beginning with G-protein coupled receptors (GPCRs), which are common drug targets. GPCRs reside in the membrane of many cell types, including neurons, providing a conduit between the outside and inside world of the cell. Chemical messengers bind to the extracellular part of the GPCR to activate it, thus engaging various signaling pathways inside the cell that modulate the cell’s activities.
In 2007, Roth’s team reported the first DREADDs, which they developed by randomly mutating the human muscarinic receptor, a GPCR that normally binds to a neurotransmitter called acetylcholine (ACh) [2]. It only took two amino acid changes to the binding pocket to ruin the receptor’s ability to bind ACh, replacing it with an affinity for an inert compound called clozapine-N-oxide (CNO), which has little to no effect in the body. “It turns out that the binding sites can be easily altered without completely destroying the receptor’s activity,” Roth says.
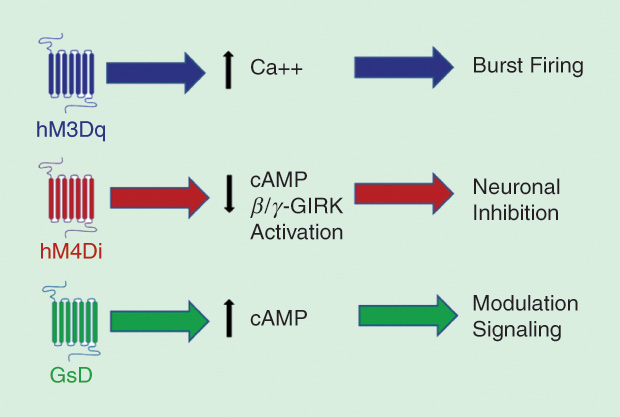
There are now four DREADDs based on the human muscarinic receptor, each activated by CNO but differing in the intracellular cascades they kick off. For example, one (hM3Dq) couples to a type of G protein that leads to calcium entry (Ca++) and bursts of electrical pulses in neurons. Another (hM4Di) quiets neural firing by opening G-protein-coupled inwardly rectifying potassium channels (GIRKs) via a fall in intracellular messenger cyclic adenosine monophosphate (cAMP). Modifying these specific pathways may have important clinical benefits (Figure 3).
“We need to tweak things, rather than go in with a big hammer,” notes Gary Aston-Jones of Rutgers University, New Jersey, who organized a 2015 meeting about the therapeutic possibilities of DREADDs (Figure 4). “DREADDs allow a more nuanced effect on the cell, which could be important for clinical efficacy.”
A fifth DREADD based on the human kappa opioid receptor, also a GPCR, has recently been developed by Roth and his colleagues [3]. This DREADD is activated by a compound called salvinorin B, which decreases neural activity, making it possible to turn on and off, on demand, neurons that express both CNO-sensitive and salvinorin-sensitive DREADDs. In mice that coexpress these DREADDs in a brain region important for mobility, CNO-induced decreases in locomotion were reversed by salvinorin B.

The chemical actuators for DREADD receptors are drug-like, in that when ingested, they reach the brain, taking minutes to exert their effects, and last for several hours. This timescale is appropriate for therapeutics; however, CNO itself may not be suitable for human use. There is some evidence in humans and nonhuman primates that enzymes turn CNO back into clozapine, a potent antipsychotic. Although the back-metabolized amounts are small, other ligands, like perlapine or one called compound 21, may be more appropriate for humans.
The receptors themselves, derived from human receptors, are not expected to alarm the human immune system. According to Aston-Jones, “The modifications made to the DREADDs are deep within the binding pocket, which means that from the outside of the cell, they look just like normal, native receptors. So they aren’t likely to generate an immunogenic response when you express them in humans.”
DREADDs for Epilepsy
The first candidates for DREADD therapy may be people with drug-resistant epilepsy. DREADDs placed in the seizure focus may permit those cells’ excitability to be remotely controlled via the DREADD ligand. Restricting treatment with DREADDs to a specific region of the brain could also limit the side effects of usual epilepsy medicines, such as sleepiness, that act beyond the seizure focus.
In 2014, Kullmann and his colleagues showed that such an approach could work in rats. DREADDs that hyperpolarized neurons were placed in the motor cortex controlling movement. Seizures were elicited by delivering a convulsant drug to the same region; however, when rats had CNO on board to activate the DREADD ahead of time, seizure frequency and severity decreased. The DREADD system also reduced seizures in rats with spontaneously occurring seizures, akin to epilepsy [4].
Patients could conceivably take a drug like perlapine to activate a DREADD that reduces firing in their hyperexcitable focus. The drug could be taken prophylactically or used as a rescue medication to prevent an ongoing seizure from devolving into the more dangerous, out-of-control status of epilepticus. However, so far, getting DREADDs into the human brain means injecting the brain with viral vectors carrying a DREADD gene. The virus enters the cell and delivers its DREADD cargo, whereupon the cell treats the gene as usual, transcribing and translating it into a protein, then shipping it off to the membrane. DREADD expression may also be restricted to a specific cell type, due to promoters coupled to the DREADD gene. For example, in Kullmann’s rat experiments, only glutamate-containing excitatory cells expressed the DREADDs. Likewise, Aston‑Jones was able to restrict DREADD expression to noradrenaline-containing neurons to study memory deficits related to Down syndrome and Alzheimer’s disease [5].
Yet viral vectors are not deal breakers for human use. They have been stripped down to mere cargo carriers, removing their ability to replicate. One type, adeno‑associated virus, is considered very safe and is currently being used in about a dozen human trials of gene therapy for nervous system conditions. Still, neurosurgery will be required. That means the first participants in a DREADD human trial may be those people with drug-resistant epilepsy who already face brain surgery to remove their seizure focus. When talking to patients about this theoretical possibility, Kullmann says the response has been positive.
Flexible Connections

People so disabled by other disorders that they are willing to undergo brain surgery for treatment may also be candidates for early DREADD trials. For example, some individuals with treatment-resistant depression can get nearly miraculous relief from DBS, in which a metal electrode is placed into the brain to deliver electrical pulses as treatment. DREADDs placed in the same region could have a similar effect. “DBS gives you a roadmap of sorts,” says Kathleen Grant of the Oregon Health and Science University, Portland (Figure 5). “With those flags in the ground, we can get into a more individualized medicine approach with DREADD work.”
This requires finding ways to target DREADDs to the desired cell type in humans. DBS activates all cell types in a region, including those that increase activity, those that decrease it, and even axonal connections just passing through. DREADDs can improve upon this, but the promoters used to target them to specific cell types in rodent studies may not work the same in monkeys or humans, Grant says, leaving researchers searching for alternatives.

DBS would also require fine-tuning activation of a particular neural circuit through different doses of ligand, which could dial DREADD activation up or down in a person, as needed. Grant and others are working on ways to visualize this activation noninvasively in nonhuman primates using brain scanning, which could eliminate dosing guesswork.
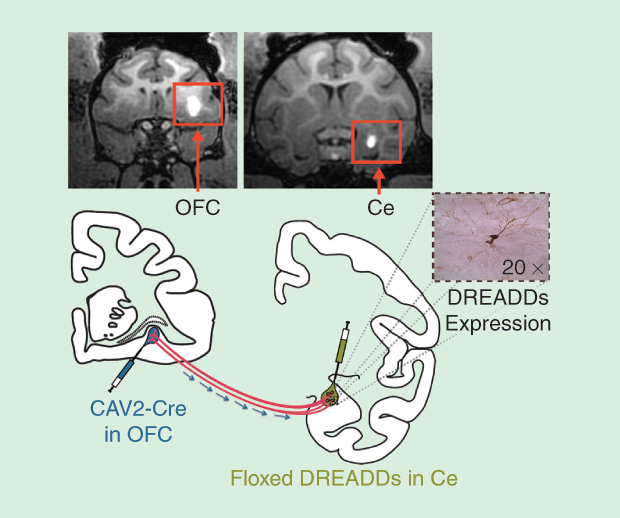
Researchers are also devising ways to express DREADDs in specific neural connections. Although neurons in the prefrontal cortex send and receive connections throughout the brain, Ned Kalin of the University of Wisconsin, Madison (Figure 6, right), has found that its connections with a region called the amygdala are important for anxiety in monkeys. He has begun to find ways to restrict DREADD expression to specific connections into and out of the amygdala (Figure 7).
For those severely disabled by anxiety, DREADDs in these circuits might allow flexible, individualized medicine. “When a person is feeling particularly disabled, we can imagine giving a medicine that would allow them to regulate that circuit through DREADDs, and then maybe not have to take the medication at another point,” Kalin explains. “That’s the science fiction, but it’s how we’re currently thinking about this.”