Globally, breast cancer is the most frequently occurring malignancy in women and the leading cause of cancer deaths, with up to half a million women dying of the disease in 2008. Early detection and accurate diagnosis of breast cancer is crucial for optimizing survival chances, with imaging technologies playing a major role. X-ray mammography (XRM) and ultrasound (US) imaging, however, suffer from nonoptimal sensitivity and specificity. Furthermore, X-ray mammography uses ionizing radiation and painful breast compression and has poor performance in dense breasts. For US imaging, interoperator dependence and poor soft tissue contrast are drawbacks.
Angiogenesis, the production of new blood vessels from the existing vasculature, has been proposed as an integral hallmark of cancer. This process results in a locally increased abnormal vascularity at tumor sites. Magnetic resonance imaging (MRI) exploits the presence of this vasculature for high-sensitivity
imaging of cancer through the visualization of extravasation of the contrast agent from the vessels. However, this method suffers from limited specificity and requires contrast agents.
By using light, the increased concentrations of hemoglobin (Hb) associated with vascularity can betray the presence of the carcinoma without recourse to contrast agents due to hemoglobin’s strong optical absorption. Photoacoustic (PA), also called optoacoustic (OA), imaging uses light as the probe energy, but measures US instead of photons. This is because laser-induced US is generated following the absorption of short light pulses. The waves propagate to the surface with low scattering and a finite (known) velocity, where they are detected using US detectors and the origin of the acoustic sources is localized. Thus, while the detection of light would have resulted in washed-out detail due to scattering, the detection of US provides high spatial resolution since it is scattered minimally in soft tissue.
Since the application of near-infrared PA (NIR-PA) for breast imaging was first suggested in 1994 [1], [2], a few breast imaging prototypes have been showcased and just over 100 patients have been imaged.
Materials and Methods
In 2005, we presented the Twente Photoacoustic Mammoscope (PAM), which uses a two-dimensional US detector matrix. PAM is built into a hospital bed on which the patient lies prone with her breast through an aperture. Under the bed, the breast is slightly compressed between a glass plate for laser illumination, with the US detector array on the opposite side (Figure 1). Pulsed light (10 ns) at 1,064 nm is used for illumination, at a radiant exposure of 10 mJcm-2 in the 35 mm2 beam, ten times lower than the maximum permissible exposure on skin. The PA signals are detected using the detector matrix with 588 unfocused elements of size 2 × 2 mm. The polyvinylidene fluoride (PVDF) elements have a central frequency of 1 MHz with a 130% bandwidth. They are distributed in a roughly circular shape with a diameter of 85 mm. US gel is used for acoustic coupling between the breast and detector, and image reconstruction is based on off-line three-dimensional acoustic backprojection.
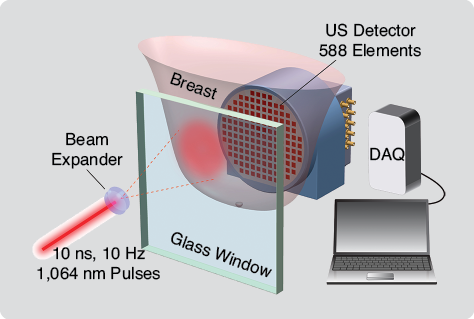
Testing of the patient safety aspects of the instrumentation was conducted prior to each study by the Medical Instrumentation Department of the Medisch Spectrum Twente (MST). The study protocols and informed consent procedures were approved by the Medical Ethics Review Board of MST. All patients followed the normal diagnostic pathway (Figure 2). When patients met the inclusion criteria for the specific study, their breasts were imaged using PAM after conventional imaging but prior to core needle biopsy to preclude imaging possible bleeding following needle insertion. In all cases, breast density was evaluated by two or three radiologists reviewing XRMs.
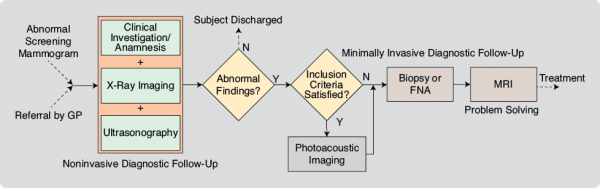
In 2007, we reported the first peer-reviewed results of NIR-PA imaging of breast cancer in patients [3]. In four of the five malignancies, the PA images with a field of view of 45 × 45 mm2 showed regions of higher intensity, which we attributed to cancer vascularization. Since these successful proof-of-principle studies, we have performed PAMmography (mammography using PAM) on 51 abnormalities comprising 41 malignancies, seven cysts, two fibroadenomas, and one chronic active inflammation. From our experiences, we make the following observations and recommendations.
PAMmography Can Visualize Infiltrating Ductal Carcinoma with High Contrast
We presented the results from an extended clinical study using PAM in 2012 [4] where we investigated the appearance of highly suspicious Breast Imaging-Reporting and Data System (BI-RADS 5) breast lesions in ten patients. The highlights of this work are as follows:
- All ten malignant lesions [infiltrating ductal carcinomas (IDCs)] were visualized in the PA images in the form of a confined region with high contrast.
- The PA contrast of the lesions varied from 3.2 to 7.0 with an average contrast of 5.0.
- In all malignancies, the PA lesion contrast was higher than the contrast in XRM.
In our latest work [5], we describe the results obtained from imaging 31 malignancies (predominantly IDC) using PAM field of view. In 30 of these cases, the lesion was identified unambiguously with PA contrasts ranging from 2.2 to 7.0 with an average contrast of 3.6. This provides compelling evidence that PA imaging has the potential for visualizing breast cancer with high contrast.
PA Lesion Contrast Is Not Dependent on Radiographic Breast Density
The average PA and XRM lesion contrasts were compared against breast density in the 2012 study on ten lesions. Based on an assessment by radiologists using BI-RADS density criteria, a two-scale classification was used (low: BI-RADS density 1, 2 and high: BI-RADS density 3, 4). In the latest study [5], the same analyses were performed on 31 lesions. The findings in the two studies are that there are no significant differences in the average PA contrast between the high- and low-density groups. Lower X-ray lesion contrasts were recorded in high-density breasts, and one lesion was missed in the 2007 study and three in the most recent study.
This early finding is very promising for the use of PAMmography in breasts rich in radiodense glandular tissue as in premenopausal subjects, where XRM is known to show reduced accuracy with sensitivity falling from around 90% to 40–60%.
Breast Malignancies Show Signature PA Presentations
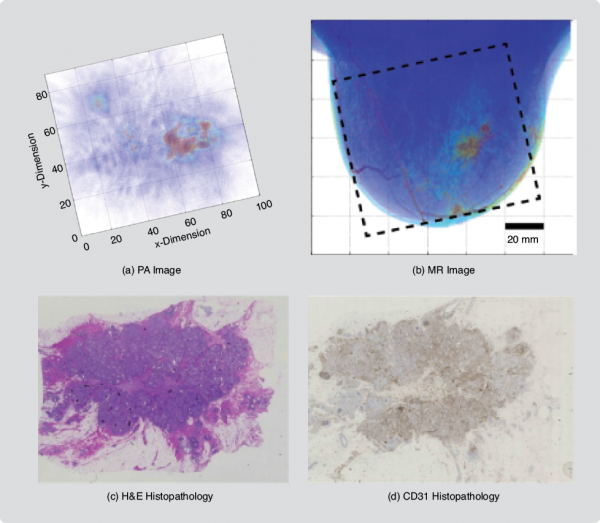
We studied the PA appearances of IDC [6] and whether these could be related to tumor vasculature. In addition to comparisons with XRM and US imaging, PA images were compared with MR images and with vascular staining in histopathology. Lesions were found to be present with various appearances similar to the contrast enhancement types reported in MRIs of breast malignancies. The following PA image patterns were found: 1) mass appearance, 2) ring appearance, and 3) nonmass appearance. MR images were available for a total of 11 cases, and correspondence was very good to excellent (Figure 3). In six cases, CD31 (a vascular stain) immunohistochemistry (IHC) on tissue sections showed good correspondence between the density and distribution of vascularity and PA image patterns. These findings are significant as they can contribute to the development of PA image descriptors as diagnostic criteria in the future.
Breast Cysts Exhibit Specific PA Appearances
The PA visibility of benign breast cysts using 1,064-nm light was also investigated [7]. It was found that depending on the absorption contrast between the cyst and breast tissue, cysts were visible as either one or two confined high-contrast areas representing the front and the front and back of the cyst, respectively. These features are the consequence of the abrupt change in the absorbed energy density and Grüneisen coefficients going across the interfaces, in combination with the limited-view geometry. Cysts can thus be mistaken for malignancies, which can also appear as one or multiple confined contrast areas.
Measuring with multiple wavelengths will be required to differentiate Hb absorption associated with malignancies, from the absorption by chromophores more characteristic for cyst fluid.
Recommendations and Outlook
On the technical side, the following points require attention in the near future:
- Multispectral excitation: This will be of utmost importance to discriminate between malignancies and benign abnormalities based on differences in Hb absorption associated with the two types of lesions.
- Quantitation of optical absorption: The PA images are developed by solving the acoustic inversion problem to recover the initial pressure distribution. The images represent absorbed energy maps—the product of the fluence and the absorption coefficient. The recovery of the absorption coefficient would permit the estimation of Hb (or other biochrome) concentrations permitting molecular imaging and in combination with 1) lead to estimation of functional measures such as oxygen saturation. Quantitative information is expected to provide an additional criterion to discriminate between benign, preinvasive, and malignant lesions. Quantitation, which involves solving the optical inverse problem, is nontrivial but is essential for accurate functional and molecular imaging.
- Tomographic geometry: A closed detection aperture with reconstructions from multiple projections as in a tomographic imaging geometry will minimize acoustic shadowing effects and provide high resolution, approaching the axial resolution of the US detectors.
- Sensitive US transducers: High detector sensitivity is required to detect small optical absorption contrasts associated with smaller tumors, or those with lower vascularity. Additionally, for faithful registration of objects with various dimensions, the bandwidth of the detector must be broad. This has consequences for sensitivity: increasing bandwidth comes at the cost of sensitivity and vice versa. Specialized US transducers are required, developed specifically for the challenging requirements in PA breast imaging.
- Multimodal imaging: Since the US detection hardware required for both techniques can be shared, PA imaging can be combined with US imaging. A dual-mode system with superposition of US images on PA images will give anatomic and structural context to functional detail. Additional parameters estimated from US imaging, such as the speed of sound and acoustic attenuation, can assist in better differentiation between certain benign lesions such as cysts from tumors and can also assist in enabling quantitative PA imaging.
On the clinical side, we believe the following to be urgent questions in PA breast imaging:
- What are the PA appearances of IDC? What are the PA features of other lesions such as infiltrating lobular carcinoma, ductal carcinoma in situ, and fibroadenomas?
- What are the pathophysiological reasons for these features? Can these features be related to angiogenic or vascular distributions in pathology? Can these appearances develop into diagnostic indicators for breast cancer?
- What are PA image descriptors of the contralateral breast and the healthy breast? Would it be possible to develop criteria for detection?
- What role will PA play in breast imaging? Is there a benefit in diagnosis, detection (screening), or both? Is there a benefit in the niche areas of neoadjuvant chemotherapy monitoring and imaging of dense breasts?
Concluding Remarks
It is clear that instrumentation for PA breast imaging is not yet as sophisticated as required. Furthermore, there are still many gaps in our understanding of the in vivo performance of the method since the early patient studies have naturally been conservative. However, the knowledge gained in the last decade in imaging patients has shown that the method has tremendous potential to impact the clinical breast cancer care model. It is a good time to be in the field of PA breast imaging.
It should be emphasized that the path from knowledge that will be gained in the future to accepted clinical practice is long, difficult, and expensive. In addition to the development of sophisticated instrumentation and early clinical assessment in the academia–hospital nexus, the role of industry in engineering and development will be important. Furthermore, when knowledge of clinical efficacy becomes clearer in the future, other stakeholders, such as organizations that make decisions regarding approval for medical devices, insurance coverages, and reimbursements, will become
increasingly important.
Competing Financial Interests
Weindelt Steenbergen and Srirang Manohar have financial interest in PA Imaging Holding B.V., which did not support the work.
Acknowledgments
We are grateful to the patients for volunteering for the study. We would like to thank Dr. Daniele Piras, Johan van Hespen, Dr. Frank van den Engh, Dr. Joost Klaase, Dr. Margreet van der Schaaf, Dr. Mariel Brinkhuis, and Maria van Boekel for helping with various aspects of the studies. The work was made possible with financial support by Agentschap NL Innovation–Oriented Research Programme (IOP) Photonic Devices under the HYMPACT Project (IPD083374). Financial contributions from the MIRA Institute for Biomedical Technology and Technical Medicine, and VICI grant 10831 (Netherlands Technology Foundation STW) are also acknowledged.
References
- A. A. Oraevsky, S. L. Jacques, R. O. Esenaliev, and F. K. Tittel, “Laser-based optoacoustic imaging in biological tissues,” Proc. SPIE—Int. Soc. Opt. Eng., vol. 2134, p. 122, July 1994.
- R. A. Kruger and P. Liu, “Photoacoustic ultrasound: Pulse production and detection in 0.5% Liposyn,” Med. Phys. vol. 21, no. 7, pp. 1179–1184, 1994.
- S. Manohar, S. E. Vaartjes, J. C. G. Van Hespen, J. M. Klaase, F. M. Van den Engh, W. Steenbergen, and T. G Van Leeuwen, “Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics,” Opt. Exp., vol. 15, no. 19, pp. 12277–12285, 2007.
- M. Heijblom, D. Piras, W. Xia, J. C. G. Van Hespen, J. M. Klaase, F. M. Van den Engh, T. G. Van Leeuwen, W. Steenbergen, and S. Manohar, “Visualizing breast cancer using the Twente Photoacoustic Mammoscope: What do we learn from twelve new patient measurements?” Opt. Exp., vol. 20, no. 11, pp. 11582–11597, 2012.
- M. Heijblom, D. Piras, F. M. Van den Engh, M. Van der Schaaf, J. M. Klaase, W. Steenbergen, and S. Manohar, “The state of the art of photoacoustic breast imaging: Results from 31 measurements on malignancies compared to conventional imaging, patient and lesion characteristics in preparation,” 2015, to be published.
- M. Heijblom, D. Piras, B. Brinkhuis, J. C. G. Van Hespen, F. M. Van den Engh, M. Van der Schaaf, J. M. Klaase, T. G. Leeuwen, W. Steenbergen, and S. Manohar, “Photoacoustic image patterns of breast carcinoma and comparisons with magnetic resonance imaging and vascular stained histopathology,” 2015, to be published.
- M. Heijblom, D. Piras, E. Maartens, E. J. J. Huisman, F. M. Van den Engh, J. M. Klaase, W. Steenbergen, and S. Manohar, “The appearance of breast cysts in forward-mode photoacoustic mammography using 1064 nm excitation.” J. Biomed. Opt., vol. 18, no. 12, pp. 126009–126009, 2013.



