Organs-on-a-chip are unlocking the secrets of preterm births and fertility problems
Female reproductive medicine may not have been entirely overlooked in the history of medical research, but it has never been given the attention that it deserves. There are signs, however, that the spotlight is turning toward the most essential of human processes.
Now, a technology that has become commonplace in drug development, disease modeling, and personalized medicine is being used put to work to target the causes of fertility and preterm birth. A microfluidic device lined with living human cells, known as an organ-on-a-chip (OoC), is being adapted to create models of the female reproductive system.
“Female reproductive medicine has not been prioritized as it should be but now that is changing,” remarks Dr. Virginia Pensabene, University academic fellow in the School of Electronic and Electrical Engineering at the Leeds Institute of Medical Research at the University of Leeds (Figure 1). “Women are more likely to go to a doctor or an oncologist than men, so their bodies should be better understood.”
“Pregnancy, birth, and menstruation are just seen as something they have to go through but they should be better understood in order to prevent disease, improve fertility, and reduce the pain women endure,” she adds. “Now, female reproductive health is becoming a key issue in medical research.”

Pensabene is a graduate in electronic engineering from the University of Pisa and holds a Ph.D. in humanoid technologies from the University of Genoa and the Italian Institute of Technology in Italy. After a role as exchange researcher at the Department of Life Science Medicine Bioscience of TWIns, Waseda University, Tokyo, Japan, she became a postdoctoral research associate at Vanderbilt University, Nashville, TN, where she began working on the development of an OoC model.
“I worked for several years on the design and development of an OoC model of the neurovascular unit, specifically focused on the blood brain barrier,” she explains. “In that period, I also completed a collaborative project with a famous neurosurgeon at the Vanderbilt Children’s hospital, Dr. Noel Tulipan. There, I learnt a lot about the properties of the fetal membranes and I started to study the maternal–fetal interaction.”
At Vanderbilt, she worked with Prof. Kevin Osteen and Prof. Kaylon Bruner-Tran, who are experts in endometriosis, the maternal–fetal interface, and reproductive toxicology in general. This led Pensabene to create an OoC model of the fetal membrane and the endometrium to study the correlation between bacterial infection and inflammation in premature birth and pregnancy failure.
“The fetal membrane changes a lot during the nine months of pregnancy,” she remarks. “We wanted to recreate it in an in vitro model and test hypotheses of reproductive toxicology. However, you can only do research on animals, not humans, so we sketched the fetal membrane on a chip.”
An organ in miniature
OoC devices, also known as tissue chips, have revolutionized the pharmaceutical industry by enabling researchers to create stable and sterile environments in which different types of cell culture can grow. Using microfluidic technology to create microchannels and micron-scale chambers, cells can grow in a space that closely approximates their natural environment.
OoCs consist of modules made of bio-compatible polymers that contain sensors, actuators, and other tools for analyzing and manipulating living cells. They can recreate a more complex physiological environment than conventional, flat plastic methods for cell culture, and have been used successfully to investigate heart, kidney, lung, liver, and many other types of cells.
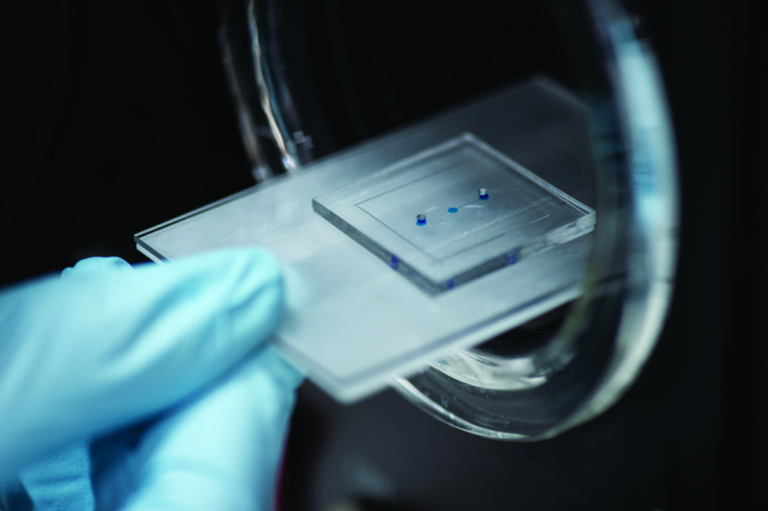
“One advantage of the OoC model is that it allows you to arrange cells in a way that mimics the organic growth of an organ,” remarks Pensabene (Figure 2). “You can stress specific cells as if they were in the body. But there are limitations. For instance, a placenta on a chip does not represent the full organ, just the placenta barrier.”
“The reproductive system has unique characteristics and what fascinates me are the changes in morphology, mechanics, cell composition, and cell response to different stimuli at different periods of life. If we consider a fertile woman, there is a continuous cycle of changes that are regulated by hormones and correspond to specific expressions of molecules and changes in the tissue characteristics of each organ.”
In pregnancy, for example, drastic changes occur to the tissues and cells in the uterus, ovaries, and endometrium, as well as the endocrine and autocrine systems that deliver hormones.
“It is evidently a great thing to model, a marvelous example of a natural cycle and of strength,” says Pensabene. “With flat plastic, we can still miniaturize by using a limited number of cells and reduced volumes, but we are completely breaking apart all the connections between different cells types, removing the matrix that keeps them together.”
Using an OoC adds the crucial third dimension.
What poses a risk to pregnancy?
OoC models have now been applied to the study of specific functions of the female reproductive tract, the organs it comprises and the intricate endocrine pathways and communication mechanisms that link them. Previously, a disease and toxicology study of this system has been difficult to perform. This has fuelled the drive for innovative platforms to support disease modeling and in vitro drug toxicity screening.
Pensabene undertook a review of recently published OoC models that recreate pathological and physiological characteristics of the female reproductive organs and tissues. The aim was to assess changes in the metabolic activity of specific cell types and the effect of exposure to hormonal treatment or chemical substances on some aspects of reproduction and fertility.
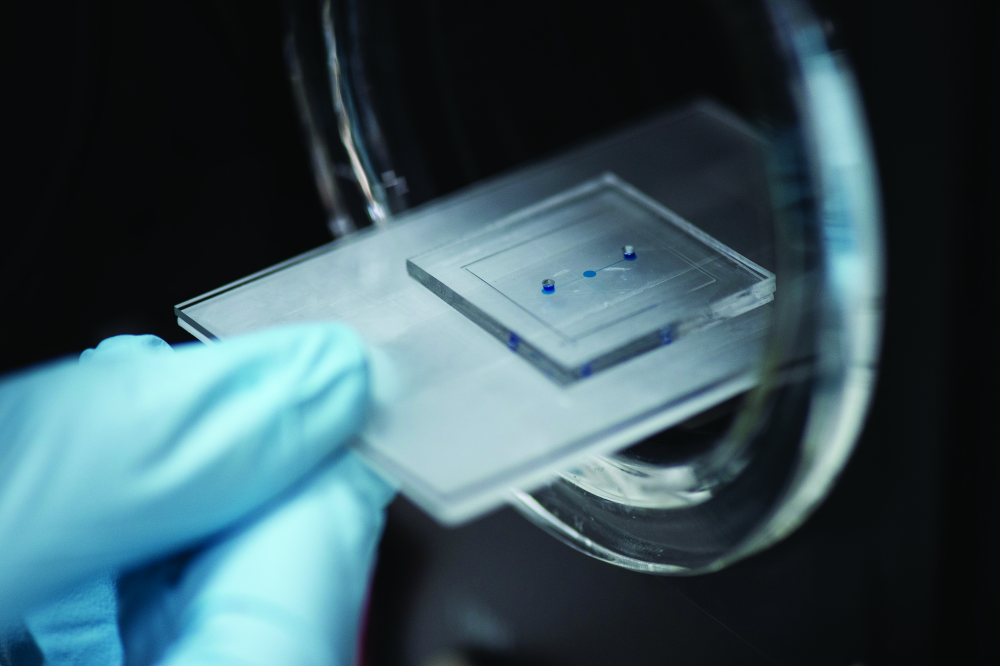
“An OoC model of this system is a very unique method to couple, in a single platform, the morphokinetic characteristics of the organs. With flat plastic, we can observe cells and their response to specific stimuli, we can use cells isolated form human patients and expose these to chemicals, microparticles or bacteria, for example, and almost any kind of stress. But with OoC, we add the 3-D structure of the organ, which is very important in these tissues and organs,” she said (Figure 3).
“The single cell types that constitute the endometrium, for example, change during the menstrual cycle in size and shape, and these affect immensely the vascularization of the tissue, the renewal of cells and the implantation success of the embryo on the endometrium.”
Using a microphysiological model of the endometrium, researchers can release specific patterns of chemicals known to be potentially harmful for fertile women and men. One example is Bisphenol A (BPA),
which has been used to make plastics and resins since the 1960s. It can be found in products as diverse as cosmetics and food packaging, and it can have a drastic effect on female fertility.

“Compounds of this nature can compete with hormones that naturally regulate our healthy cycle and can thus disrupt the physiological functions of the whole system,” Pensabene explains. “With a microfluidic system, stimulation with toxicants can be dynamically controlled and we can dissect the specific response of the different cells, while keeping intact the microphysiological characteristics. These OoC models have typical volumes that are comparable to the 96-well reaction plates commonly used as gold standard in high-throughput pharmaceutical studies.”
For one of her own projects, Pensabene developed a fetal membrane OoC to study the effect of the bacteria propagation through these membranes and to assess whether the infection could alter the membrane structure and cause premature birth. She has been using this model to test the hypothesis that only the concomitant presence of inflammation and infection leads to prelabor rupture of membranes (Figure 4).

“It has previously been difficult to perform disease and toxicology studies of this system due to ethical concerns,” she notes. “Studying placenta function in a pregnant woman is unacceptable, as is collecting tissues or ovarian biopsies from healthy women.”
A web of research
Pensabene’s own work is just part of the growing use of OoCs to better understand the female reproductive system. She points to the work of Teresa Woodruff at Northwestern University in Chicago, IL, and
Dr. Shuo Xiao, assistant professor with the Reproductive Health & Toxicology Laboratory in the Department of Environmental Health Sciences at the University of South Carolina, who have developed five organs—the Fallopian tubes, uterus, cervix, ovaries, and liver—linked together by a blood-like liquid carrying hormones, cell signaling molecules, and drugs.
At University Hospital Tübingen, Dr. Peter Loskill has developed a patient-specific cervix-on-a-chip model that allows mechanistic research, disease modeling, and drug testing. At the Vienna University of Technology, Prof. Peter Ertl was one of the first to produce an artificial placenta model that very closely resembles the natural organ.
The belief is that OoCs will help researchers to understand the complex physiology of the reproductive systems in more detail. For example, the research may shed light on how the endometrium communicates with the embryo in the preimplantation stage, which would unlock tailored therapeutic treatment for women with fertility problems. A better understanding of the ovaries might provide insights into their dysfunction and, potentially, the causes of ovarian cancer.
“Miscarriage and preterm birth are being looked at as a disorder for the first time,” says Pensabene. “We need to look at it like any other disease. OoC models have been used for many organs, but the female reproductive systems is a brand-new area for this technology.”