Questions about safety have pointed to some new directions in research
Carbon nanotubes (CNTs), those tiny cylindrical configurations of pure carbon that have been finding myriad applications in a wide variety of fields, have been the subject of headlines for well over a decade for their potential uses in biological research and medical treatment. Progress toward those goals has been slowed by questions about the safety of the tiny particles when injected directly into the body, where they can sometimes accumulate in certain organs with unknown longterm effects.
However, many teams working with the novel materials, which can have a number of potentially significant advantages over currently used materials, are continuing to make progress toward applications that could be of real clinical significance within the next few years. These include electrically conductive fibers made of CNTs that could be used to repair heart defects and other clinical issues, drug delivery systems that might be able to target certain organs, and several other promising avenues of ongoing research.
Heart bridge
Researchers at the Texas Heart Institute (THI), Rice University in Houston, and collaborators atother institutions have been developing one of the most promising of these technologies, a kind of fiber made of millions of CNTs embedded in a polymer matrix. The fiber is similar enough to the silk thread commonly used in sutures that doctors can readily adapt to using the new material, the researchers say (Figure 1).

In their initial animal tests, the fibers were able to provide an electrical bridge across damaged sections of heart muscle, thus restoring normal function of the electrical signals that control the heart’s contractions. The hope is that such a mechanical bridging could someday restore normal function to hearts damaged through myocardial infarction or other injury by providing a conductive link to bypass scar tissue that is nonconductive (Figure 2).
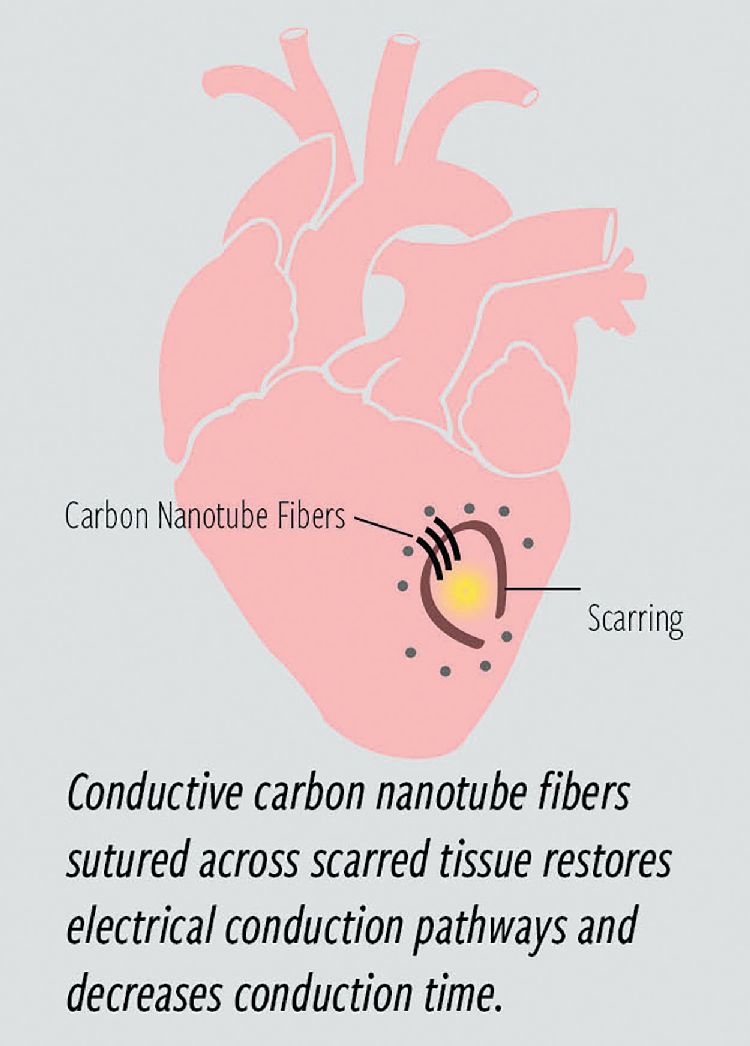
Instead of having to resort to defibrillation using electrical shocks for a heart whose damaged portions cannot conduct the normal control pulses, this would allow an actual restoration of normal functioning of the heart by providing a new conduction pathway, as long as the suture remains in place and maintains its conductive characteristics. So far, tests in large animals including sheep and pigs have been in place for a few months without any sign of degradation, says Dr. Mehdi Razavi, a cardiologist and director of Electrophysiology Clinical Research and Innovations at THI, who has been coleading the research (Figure 3).
Razavi says that today there is no available treatment that can actually address the underlying problem of scarred, nonconductive tissue in the heart muscle, which leads to ventricular arrhythmias. This disordered firing of impulses from the lower chambers of the heart can result from scarring caused by heart attacks, congestive heart failure or dilated cardiomyopathy, he says.

A cardiac surgeon himself, Razavi says that when he works with the new fibers, they handle very much like traditional silk-based suture threads, although for now the CNT threads are thicker (Figure 4). “When I use it, it feels like I’m tying a silk suture,” he says.
Cardiac electrical disturbances are one of the leading causes of death in the United States, so any treatment that could make a difference for such patients could have a significant impact. So far drugs have been one of the few available treatments, and like all drugs, are subject to some side effects. Other treatments include ablation of the scarred tissue, but that reduces the thickness and strength of the heart wall itself. The CNT fibers—with tens of millions of the minuscule tubes, each just a few nanometers across—are embedded in a neutral, nontoxic polymer coating, and so far have shown no adverse reactions in rodents or larger animals.

One crucial engineering step that remains is to develop catheter-based tools capable of delivering the fibers and sewing them in place to the heart muscle, in order to avoid the need for invasive openchest surgery. Razavi is hopeful that the work will reach the point of initial human clinical trials within three to four years. After an initial research paper on the process received widespread attention last year, the group has expanded their work and is publishing several new papers on the progress in late 2020 and early 2021.
One of these new publications describes extensive testing of the safety of the fibers in the various animal models over extended periods. “There was no change at all” in electrical conduction over an extended period, Razavi says, indicating there was no degradation of the material. This has been a major concern, and research including a study published last year found that CNTs in the body have the potential to cause cancers such as mesothelioma, just as do asbestos fibers, which have similar dimensions. But this new research so far shows that when CNTs are embedded in the polymer, those fibers can remain intact in the body for extended periods and show no such adverse effects.
The robustness of these materials is obviously crucial, if they are to be sewn in place in a heart that beats about a hundred thousand times a day, year after year. Accelerated aging tests are also underway to assess the long-term performance.
“We don’t see any evidence of toxicity,” adds Dr. Mark McCauley, an assistant professor at the University of Illinois at Chicago College of Medicine, who initially worked on the development of the CNT fibers while a postdoc at THI. No adverse physiological signs have shown up in the testing so far, he says.
McCauley adds that besides the immediate applications to cardiac conditions that the team has been focusing on, these same CNT fibers might find other clinical applications where a flexible, conductive, and nontoxic material is needed. These include possibilities in the area of restoring conduction in neuromuscular junctions, he says. For example, electrical signals from functional muscles on unaffected parts of the body of a partially paralyzed stroke victim might be connected to those in the afflicted areas, perhaps restoring some functionality. Or severed neural connections might someday be bridged by such materials, and potentially aid in restoring a natural voice to people with partially paralyzed vocal cords. Other possible applications might be as replacements for the stiffer metallic fibers used in cochlear implants, he suggests.
Drug delivery
Other research teams have continued to develop a variety of other potential applications of CNTs to biomedical research and treatment. Among them, a team based at the Maine Medical Center and a company called Biotact have been working to develop CNT-based drug delivery systems, and have shown some promising results.
Initially, the work was focused on in vivo drug delivery, explains Aaron Tasset (Figure 5), a member of the research team and coauthor of a paper on their results published in 2019. But because of safety concerns, for the moment the company is focusing on in vitro applications, which would not require such a long regulatory process before potentially being implemented. For example, the materials might be used to deliver the messenger RNA (mRNA) or clustered regularly interspaced short palindromic repeats, a DNA sequence used in genetic engineering (CRISPR) molecules or other vectors used for delivering genetic modifications into cells, before then injecting the modified cells back into the body. Since the CNTs would never be injected into the body, the safety issues of concentration of those materials in certain organs would not apply.

“The big issue is the retention inside the body,” Tasset explains. Although their studies didn’t show any negative effects in animals, he says, there is some retention of the CNTs in certain organ systems when they are injected directly into the body. In any case, the open questions regarding these effects will delay any the U.S. Food and Drug Administration (FDA) approval for such applications, hence Biotact’s pivot to uses that don’t require injection of the materials into the body.
Multiwalled nanotubes, which are essentially a set of nested tubes, do hold great promise as drug delivery agents because of their very large drug-binding surface area, the team says, if these safety issues can be addressed. Tasset is confident they will be able to address these safety concerns. The company’s longer-term hope is that this retention property can be turned into an advantage for treating certain kinds of specific conditions, he says. Since the particles tend to accumulate preferentially in certain parts of the body, such as the spleen, liver, and bones, the idea is to make use of that by using the CNTs to deliver drugs or treatments directly to those organs when they are needed.
Safety first
Research continues around the world on a wide variety of other potential uses for these extraordinary tiny molecules with their versatile properties. Another area of promising biomedical applications for CNTs is as contrast agents for some medical imaging systems. There is also promising work on using them as sensors by functionalizing the CNTs with certain chemical groups, for example, to detect specific proteins for diagnostic purposes.
Other groups have developed X-ray emitters based on CNTs, which might allow for precisely targeted internal imaging. Alternatively, some research has used tiny nanosprings coated with CNTs as force sensors in a skin patch that can accurately measure the force of arterial pulses. Such a sensor might someday be coupled to a CNT-based device that could apply counterpressure in response to signals picked up by the sensor.
Because of the concerns about the physical effects of certain forms of long CNTs in the body, the International Chemical Secretariat earlier this year added CNTs to a list of “substitute it now” materials that need to be limited or phased out within the European Union (EU), a ruling that has drawn loud protests from various scientific groups. The critics point out that the ruling fails to distinguish between certain specific sizes and types of CNTs that have been shown to have harmful effects, and the many others that have not. The ruling, if it stands, could seriously curtail research in the field throughout the EU.
But elsewhere around the world, the research is going on at a rapid pace, in a wide variety of different approaches and applications. While none are yet in actual human clinical use, the field continues to show enormous promise. As a review article in Current Medical Chemistry pointed out last year, “the unique mechanical, electrical, thermal, chemical, and optical properties of carbon-based materials like fullerenes, graphene, CNTs and their derivatives made them widely used materials for various applications including biomedicine,” citing advances in cancer therapy, drug delivery, bio-sensing, cell and tissue imaging and regenerative medicine, while also citing the safety challenges associated with them.
All in all, after a burst of excitement early this century over the great biomedical potential of CNTs, progress has taken longer than many expected, mainly because of the very real and valid safety concerns. Now, though, with those concerns recognized and better understood, the field seems to be making real and substantial progress.