Magnetic resonance imaging (MRI) is a ubiquitous tool used in clinical settings around the world to provide detailed three-dimensional information on the internal anatomy and physiology of human patients without the use of ionizing radiation, which is the primary safety concern associated with computed tomography. This information is obtained noninvasively and can be used in the diagnosis of pathological conditions as well as the monitoring of treatments.
MRI employs a strong (and ideally uniform) static magnetic field to align the spins of protons within the body. The application of a radio-frequency (RF) excitation pulse perturbs the protons away from the direction of the magnetic field. Removal of the RF excitation allows the protons to return to their aligned state. The energy released during relaxation is tissue specific, which allows clinicians to identify differences between multiple tissues.
The Need for Virtual Humans in an MRI Setting
Despite the absence of ionizing radiation, MRI is not without safety concerns. The strong electromagnetic (EM) fields involved (typical clinical values are 1.5 T and 3.0 T, pulsed at 64 and 128 MHz, respectively) present risks to patients with implanted magnetic or conductive medical devices. These risks include magnetically induced displacements and torques and localized heating due to RF energy exposure. Therefore, all devices are classified as either MR-safe, MR-conditional, or MR-unsafe, according to ASTM standard F2503-13. Those patients whose devices carry MR-unsafe labels are automatically excluded from receiving an MRI procedure, while patients with MR-conditional devices may receive the procedure if scan conditions prescribed by the manufacturer are met.
In addition to safety and regulatory concerns related to MRI, new scanner designs that maximize the received signal-to-noise ratio while reducing patient exposure and time and motion artifacts are continuously being proposed and evaluated. New techniques, field strengths, and specialized applications of MRI are being actively researched to enable safer, more accurate scanning procedures with better spatial resolution. However, limited cost and time resources preclude full experimentation, optimization, and safety characterization of many new ideas. For these reasons, it is necessary to have available anatomically accurate virtual humans, also known as computational phantoms, that may be used in high-fidelity numerical simulations of MRI designs and protocols.
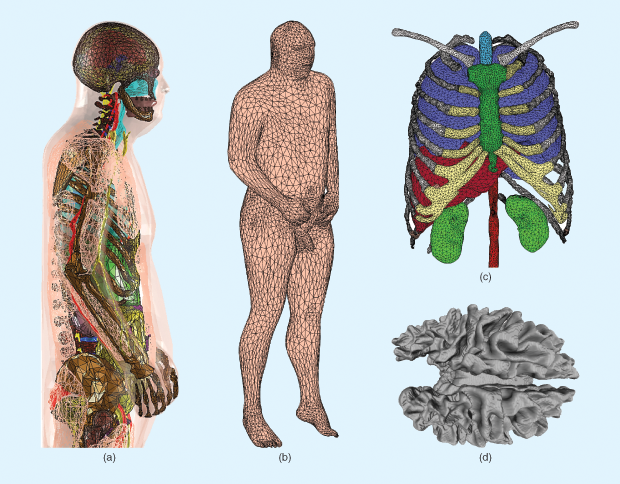
One data set of virtual humans, the American Virtual Family (shown in Figure 1), has been constructed using data assembled, maintained and provided by the U.S. National Library of Medicine’s Visible Human Project (VHP). The American Virtual Family is currently composed of two virtual humans (one male and one female) constructed using triangular surface meshes that represent the geometric shape of various tissue structures within the body. Creation of the VHP-female and VHP-male computer-aided design (CAD) models was accomplished using standard segmentation and mesh-processing techniques [1]. The American Virtual Family has been seamlessly incorporated into ANSYS HFSS simulators and Maxwell3D rendering software, which employ the finite-element method to numerically solve Maxwell’s equations and have shown a high degree of utility across a range of applications [2]–[7].
Use of Virtual Humans for Design and Safety Considerations
Computational modeling with virtual humans combines mathematics, physics, and computer science to study the behaviors and reactions of complex bioelectromagnetic and multiphysical problems in silico. Therefore, these models offer a cost- effective means of examining a host of potential designs, optimizing those that appear most promising, and provide qualitative evaluations on safety that may be further validated experimentally.
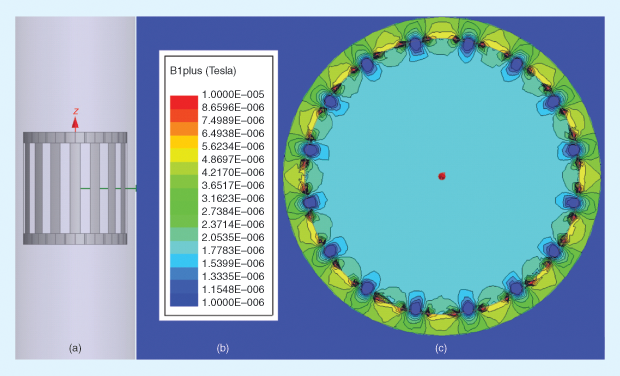
Computational modeling is increasingly being used by researchers, regulatory agencies, and MRI developers to understand not only the EM environment inside unloaded (empty) coils, as shown in Figure 2, but also the interactions between the EM field and the human body under realistic (loaded-coil) conditions. These studies seek to provide estimates of energy absorption and temperature rise during an MRI procedure. For example, specific absorption rate (SAR) is a measure of the power deposition per unit mass and is commonly accepted as a surrogate for thermal temperature rise. Keeping within established SAR safety limits, as regulated by the U.S. Federal Communications Commission, minimizes the risk for tissue damage due to thermal effects.
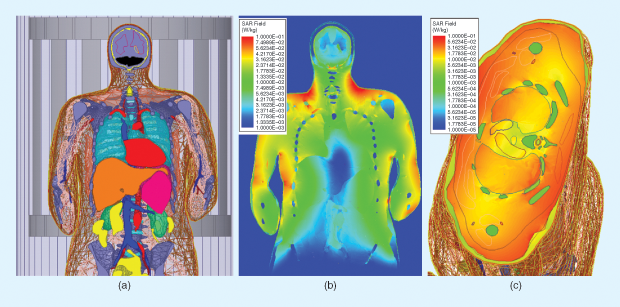
Using computational modeling and simulation tools in combination with virtual humans for initial assessment of SAR resulting from a particular device (such as an MRI coil) has a distinct advantage: SAR may be assessed at any point or within any volume included in the virtual space. Figure 3 illustrates this for both the coronal and axial body planes.
Future Virtual Human Developments
Further advances in virtual human complexity, both in terms of material modeling and higher levels of anatomical accuracy, can be achieved as solver robustness increases and computational costs decrease. For example, preliminary work constructing a realistic breathing sequence as part of the VHP-female model has already been accomplished [1]. Similar modeling is anticipated to capture other physiological features, such as pulsatile blood flow and advanced posing. Incorporating new methodologies that simulate fibrous tissues, such as white matter and musculature, may provide greater insight into these highly anisotropic structures. These developments will provide medical practitioners and patients with superior data, enabling more informed and effective healthcare decisions.
References
- H. Tankaria, X. J. Jackson, R. Borwankar, G. N. K. Srichandhru, A. L. Tran, J. Yanamadala, G. M. Noetscher, A. Nazarian, S. Louie, and S. N. Makarov, “VHP-Female full-body human CAD model for cross-platform FEM simulations—Recent development and validations,” in Proc. 38th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC 2016), Orlando, FL, 2016, pp. 2232–2235.
- S. N. Makarov, J. Yanamadala, M. W. Piazza, A. M. Helderman, N. S. Thang, E. H. Burnham, and A. Pascual-Leone, “Preliminary upper estimate of peak currents in transcranial magnetic stimulation at distant locations from a TMS coil,” IEEE Trans. Biomed. Eng., vol. 63, no. 9, pp. 1944–1955, Sept. 2016.
- G. M. Noetscher, J. Yanamadala, H. V. Tankaria, X. J. Jackson, S. Louie, A. Prokop, A. Nazarian, and S. Makarov, “VHP-Female CAD human model family for antenna modeling,” in Proc. 2016 IEEE Int. Symp. Antennas and Propagation/USNC-URSI National Radio Science Meeting, 2016, pp. 1629–1630.
- M. Kozlov, G. M. Noetscher, A. Nazarian, and S. N. Makarov, “Comparative analysis of different hip implants within a realistic human model located inside a 1.5T MRI whole body RF coil,” in Proc. 37th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society, Milan, Italy, 2015, pp. 7913–7916.
- M. Kozlov, P.-L. Bazin, H. E. Möller, and N. Weiskopf, “Influence of cerebrospinal fluid on specific absorption rate generated by 300MHz MRI transmit array,” in Proc. 10th European Conf. Antennas and Propagation, Davos, Switzerland, 2016, pp. 1–5.
- A. Venkatasubramanian and B. Gifford, “Modeling and design of antennas for implantable telemetry applications,” in Proc. 2016 38th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, 2016, pp. 6469 –6472.
- G. M. Noetscher, J. Yanamadala, S. N. Makarov, and A. Pascual- Leone, “Comparison of cephalic and extracephalic montages for transcranial direct current stimulation—A numerical study,” IEEE Trans. Biomed. Eng., vol. 61, no. 9, pp. 2488–2498, Sept. 2014.
- S. N. Makarov, A. Pascual-Leone, and A. Nummenmaa, “Modeling fiber-like conductivity structures via the boundary element method using thin-wire approximation. I construction of basis functions,” in Proc. 2016 38th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, 2016, pp. 6473– 6476.



