With the advent of additive manufacturing and a flurry of new-generation, three-dimensional (3D) printers that hit the market in the early 2000s, biomedical innovators began envisioning the technology as a way to replace damaged or diseased tissue and organs with on-demand, printed parts [1]–[3]. The path from vision to reality was not quite as quick or easy as many anticipated, but research groups today are making headway to keep the technology moving toward its goal.
“When the whole field started, we all believed that we were going to bioprint a heart in two years, and that obviously didn’t happen. Further research is needed to address the complexity of biology, but now we have much more powerful tools to get us closer to that objective of printing functional tissues and organs,” said Riccardo Levato, Ph.D., biofabrication researcher at the UMC Utrecht and coordinator of a large European bioprinting effort called ENLIGHT that is employing a new bioprinting technology to generate a living model of pancreatic tissue by 2025 (Figure 1).
In addition to the multi-institution, multicompany ENLIGHT initiative, pioneering work is under way in a number of other research groups that are also pushing the field forward. Some, such as a group at Carnegie Mellon University (CMU) in Pittsburgh, PA, have developed advanced bioprinting techniques well-suited for generating soft-tissue structures. Others, including a team at Wake Forest School of Medicine in Winston-Salem, NC, are working on methods to improve key steps in the bioprinting process, and help pave the way for replacement tissues and organs constructed of a patient’s own cells.

Heart model, valve printing
The work at CMU centers around a technique called Freeform Reversible Embedding of Suspended Hydrogels (FRESH). FRESH had its start back in 2010 when Adam Feinberg, Ph.D., first joined CMU as an assistant professor of biomedical engineering and materials science and engineering (Figure 2). At that time, researchers were beginning to explore how to use a 3D printer to build biological tissues, instead of the plastic or metal objects typically made. For printing plastic, a thin filament is melted and then extruded precisely via a nozzle one thin layer at a time so that each new layer bonds with the former as it rapidly cools and hardens. “Generating tissue is very different, because printing uses cells and bioinks (biocompatible polymers) that are soft, deformable, don’t hold their shape very well, and are temperature-sensitive,” said Feinberg, now full professor and principal investigator of CMU’s Regenerative Biomaterials and Therapeutics Group.

The group’s solution was to print not in air, but inside of a water-based hydrogel with a consistency similar to that of hair gel, so that the extrusion needle could move through it freely, yet was solid enough to provide support for the extruded bioink and cells (Figure 3). Graduate student Thomas “T. J.” Hinton came up with the right mix: a gelatin-microparticle slurry that he was originally able to make using a particular type of food blender [4]. The hydrogel had additional benefits, including biocompatibility with the printing of a wide range of cell types and other biological materials important for growing tissues. Using gelatin enabled the hydrogel support to be temperature-sensitive, allowing researchers to print at room temperature, and when completed, warm it to body temperature to liquify the hydrogel and pull out the printed item; and an enclosed environment that could provide sterile printed conditions.
After showing that the approach worked for small objects about the size of a grape, the group in 2020 demonstrated its fundamental scalability by printing a physical model of a full-size human heart [6]. Made of the polymer alginate, the model was built to order using magnetic resonance images of a real heart. Because alginate feels and responds like heart tissue, such a model also has practical value, Feinberg said, noting that surgeons can prepare for a challenging operation by cutting, suturing, and cauterizing this very realistic replica of the patient’s own heart.
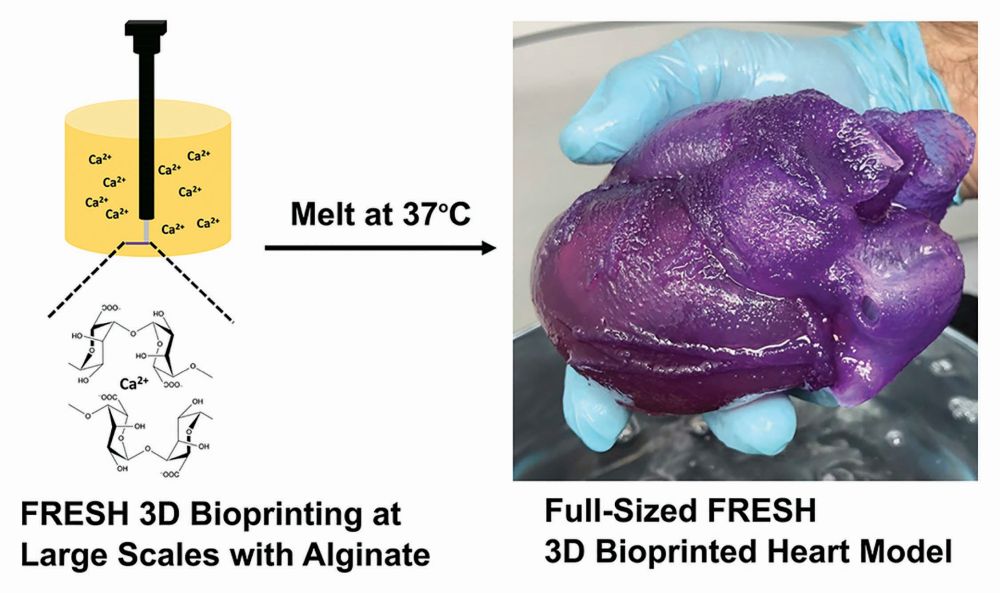
In 2018, the spinoff company FluidForm of Acton, MA, began expanding on the CMU group’s work and starting commercialization. As an example, the company is building on collagen-based, heart-valve research [7] to develop a higher performing alternative to the porcine-derived valves used in the clinic today. “Our FluidForm team is also working on a small model of the human ventricle, which is about the size of your pinky finger, and made out of human heart muscle cells created using a patient’s own stem cells,” Feinberg said. The ventricle model will allow pharmaceutical companies to test candidate drugs and screen out poor performers earlier in the typically long and costly drug-development process.
“Even with all of these projects ongoing, there’s only so much that we can do, so FluidForm has also begun marketing the supportive hydrogel, now named LifeSupport, for the research market,” Feinberg said. “This amplifies our contribution to the field, because it allows any researcher out there to have access to the technology, hopefully innovate using our platform, and do really cool and high-impact science with it.”
Quest for a living pancreas
The ENLIGHT group is also working on a bioprinted pharmacologic model, but its objective is to construct tissue with an integrated network of pancreatic islets connected by a vascularized network. “The idea is to create multiple aspects of pancreatic tissue with the printer, so we can study the biodistribution of diabetic therapeutics and how they interact with not only the endocrine islets, including different components of the islets, such as alpha, beta, and delta cells, but also with the vasculature of the pancreas,” Levato said. In addition, he noted, the group will also focus on printing tissue that will allow for study of drug effects and toxicities on the pancreas long-term, something that is not possible with currently used—and short-lived—explanted islets.
Central to the project is a volumetric bioprinting method [8] developed by Levato’s group at UMC Utrecht with collaborators at the Laboratory of Applied Photonics Devices from the Swiss Federal Institute of Technology (EPFL) in Lausanne, Switzerland. Different from the layer-by-layer, 3D-printing approach, volumetric bioprinting uses a container of hydrogel in which biocompatible photopolymers are suspended (Figure 4). The printer spins the container of gel while a beam of laser light penetrates it. Using a digital model of the pancreas as a guide and a single source of laser light, an algorithm precisely and sequentially directs light from multiple angles into the gel, and those locations receiving sufficient collective intensities of light react and solidify. “In other words, one single projection of light from one angle doesn’t provide enough energy for the gel to solidify, but the combination of many projections from different angles does the trick,” Levato described. As if by magic, a 3D object begins to appear in the gel, and once completed, the remaining unreactive hydrogel can simply be washed away to leave the object behind.

A major benefit of volumetric bioprinting is its speed. “In a nutshell, we can print anything of the size of several cm3 with our printer, with the biggest printed at the moment at 4 cm3 and that was done in less than 25 seconds,” he said, noting that a standard layer-by-layer 3D printer would need about 2 hours to print the same thing, and even an exceptionally fast version would require about 10–20 minutes. In addition, volumetric bioprinting allows for the generation of exceedingly complicated structures and vascular networks (Figures 5 and 6) without the need for supports that would typically be required for traditional 3D printing. “That means it’s easier with volumetric bioprinting, and we can also get a bit of a higher resolution,” he remarked.
Like other bioprinting approaches, volumetric bioprinting is an important step toward making tissue, but it doesn’t yield a living product, Levato reminded. “The object we’re printing may look like a tissue anatomically, but until the cells mature and connect with each other—until the biology kicks in—it doesn’t yet become a tissue.” The ENLIGHT consortium is beginning work on that aspect now. Specifically, consortium partners ETH Zurich, AstraZeneca, and the University of Naples have taken on the task of designing improved differentiation routes to generate progenitor cells, as well as the different subsets of endocrine cells.

As that proceeds, UMC Utrecht and other consortium partners, including the companies Readily3D and Rousselot Biomedical, are working to improve the volumetric bioprinting technology and boost the resolution even more, and to optimize the photopolymer-containing gel to encourage highly efficient and effective tissue maturation. “The goal by 2025—just four years from now—is to have at least a 1 cm3 construct with islets and vascularization in place, and that will allow us to test a series of new compounds produced by our pharmaceutical partners, and also a mouse transplantation of the tissue to show proof-of-concept that this technology can be used for in vivo applications,” Levato said. “While ENLIGHT has a most ambitious goal, we are confident that we can do it.”
Quicker tissue generation
One of the ultimate objectives in bioprinting is to generate replacement organs made of the patient’s cells, but as Levato pointed out, printing something that has the shape of living tissue is not the same as creating real and functional living tissue. “This is important, because 1) we have a donated-organ shortage; and 2) 20% of patients who are on the waiting lists have already undergone transplantation, but despite the many drugs used to suppress the immune system, their bodies rejected the organs so the patients are back on the list,” said Ashkan Shafiee, Ph.D., research scientist at Wake Forest Institute for Regenerative Medicine (Figure 7). “For these reasons, it is best to transplant organs made of the patients’ own cells using a process known as autologous tissue engineering.”
To develop autologous organs, however, time is of the essence, Shafiee said. “But organ engineering is a very long process: You have to do the biopsy, proliferate the cells, do the biofabrication of the structure, go through tissue maturation, and then finally, you can say, OK, this organ is ready for transplantation. So, if we can accelerate any single step in this long procedure, it can benefit the patient at the end.”
To that end, Shafiee’s group is focusing its efforts on understanding the physics of tissue engineering and the underlying science of bioprinting. They developed a model based on cellular-adhesion molecules, and calculated the energy and force required by these proteins to elicit tissue formation [9], [10]. Using data derived from their model, they were able to hasten post-printing tissue maturation, and accordingly, develop a new mechanotransduction-based bioprinter [11]. This bioprinter functions by essentially pushing cells together to heighten cell-to-cell interplay, which in turn elicits faster tissue generation. “Here, cellular bioinks are made of human cells without any biomaterials or polymers, thereby mimicking nature. Consider the point of contact between two adjacent printed cellular bioinks, which would be an imaginary line between two of the commonly used cylindrical bioinks. The cell on each side of the line or point of contact would sense each other, secrete cell-adhesion molecules, and begin bonding,” he described. “Once they are attached to each other, they bring a few more adjacent cells into proximity, whereupon the process repeats itself. So, it’s like a zipper, wherein each link engages and closes, causing the next link to engage and close, and so on.”

To push the cells together, the researchers added grooves to the bioprinter to impart an external force onto the cells in the printed object. For instance, a row of adjacent cylindrical bioinks placed on a flat surface touch one another, but if the surface is then bent into a U-shape, neighboring cylinders at the bottom of the U are pressed together more tightly. This could help cells overcome some of the repulsive forces that decelerate tissue formation, and the resulting, more forceful contact effectively pulls up the zipper faster, he explained. Shafiee’s group then compared the zipping (or cell fusion) time required when using a normal bioprinter to that required with the mechanotransduction-based counterpart. “For a normal bioprinter, fusion started 5.23 hours after the cells first sensed each other, but with mechanotransduction, it took only 1.12 hours, which was almost one-fifth the time,” he said. “Besides accelerating the process, we also confirmed that the added external force does not damage the cells.”
Shafiee and his group are now tweaking the printer so that they can fine-tune the external force and determine exactly how much of it is optimal for various cell types, which may have different deformative, or viscoelastic, tolerances. They also hope to study applied forces to determine whether the bioprinter could potentially be used to print structures inside the body during surgery or even to print food for astronauts in space, he said. “I have been amazed at how the integration of physics and biology can create so many things, even beyond regenerative medicine. We hope that wherever external forces are involved in tissue formation, we can use the zipper model and mechanotransduction-based bioprinting to complete the task faster.”
Following life’s path
Levato, Feinberg, and Shafiee each noted that progress in the field will require contributions from multidisciplinary research groups in academia and industry, all working simultaneously to mimic something the body does naturally: create living, functional human tissue.
“3D bioprinting is an overhyped technology, and yes, people are out there even today saying they can print organs, but it’s still at least a decade out,” Feinberg said. “However, there’s a lot of impactful stuff we’re going to do before then (in terms of) building functional human tissues, and we’re working toward the goal of integration into full organs.”
Levato agreed. “The field was overconfident about organ bioprinting in the past and underestimated biology, but we have now much more powerful tools to get us closer to that objective, so while considerable work remains ahead, I am confident.”
“People have been excited only about the technology of bioprinting, but have undervalued the rich science behind the scenes. Therefore, at some point, the achievements were way behind expectations,” Shafiee added. “Once we learn to better appreciate how Mother Nature does things, we can apply that knowledge to the technology to help attain our goal.”
References
- V. Mironov, V. Kasyanov, C. Drake, and R. R. Markwald, “Organ printing: Promises and challenges,” Regen. Med., vol. 3, no. 1, pp. 93–103, Jan. 2008.
- X. Wang, Y. Yan, and R. Zhang, “Recent trends and challenges in complex organ manufacturing,” Tissue Eng. Part B, Rev., vol. 16, no. 2, pp. 189–197, Apr. 2010.
- M. Shaer, “Soon, your doctor could print a human organ on demand,” Smithsonian Mag., May 2015. Accessed: Jun. 16, 2021. [Online]. Available: https://www.smithsonianmag.com/innovation/soon-doctor-print-human-organ-on-demand-180954951/
- T. J. Hinton et al., “Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels,” Sci. Adv., vol. 1, no. 9, e1500758, Oct. 23, 2015.
- D. J. Shiwarski, A. R. Hudson, J. W. Tashman, and
A. W. Feinberg, “Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication,” APL Bioeng., vol. 5, art. no. 010904, 2021, Accessed: Jun. 16, 2021. [Online]. Available: https://doi. org/10.1063/5.0032777 - E. Mirdamadi, J. W. Tashman, D. J. Shiwarski, R. N. Palchesko, and A. W. Feinberg, “FRESH 3D bioprinting a full-size model of the human heart,” ACS Biomater. Sci. Eng., vol. 6, no. 11, pp. 6453–6459, Nov. 9, 2020.
- A. Lee et al., “3D bioprinting of collagen to rebuild components of the human heart,” Science, vol. 365, no. 6452, pp. 482–487, Aug. 2, 2019.
- P. N. Bernal et al., “Volumetric bioprinting of complex living‐tissue constructs within seconds,” Adv. Mater., Article no. 1904209, Oct. 18, 2019. Accessed: Jun. 16, 2021. [Online]. Available: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201904209
- A. Shafiee, C. Norotte, and E. Ghadiri, “Cellular bioink surface tension: A tunable biophysical parameter for faster maturation of bioprinted tissue,” Bioprinting, vol. 8, no. 13, pp. 13–21, Dec. 2017.
- A. Shafiee, E. Ghadiri, D. Williams, and A. Atala, “Physics of cellular self-assembly: A microscopic model and mathematical framework for faster maturation of bioprinted tissues,” Bioprinting, vol. 14, e00047, Jun. 2019.
- A. Shafiee, J. Kassis, A. Atala, and E. Ghadiri, “Acceleration of tissue maturation by mechanotransduction-based bioprinting,” Phys. Rev. Res., vol. 3, no. 1, Jan.–Mar., 2021, Art. no. 013008.



