New virus detectors using LAMP, fitness trackers, and air samplers offer faster, cheaper, yet reliable options now and for the next pandemic
Earlier, better detection of SARS-CoV-2 infection and exposure is a necessary part of battling COVID-19, and many research groups are working on new methods to do it.
One group at the University of California Santa Barbara (UCSB) is developing an inexpensive, 25-minute, smartphone-enabled test that works rather like a portable polymerase chain reaction (PCR) machine to pick up even very small quantities of virus. Another at Scripps Research Translational Institute, La Jolla, CA, USA, is focusing on a smartphone app to spot early, silent symptoms of COVID-19. In a different approach, a team at Yale University, New Haven, CT, USA, is developing a device to determine exposure levels in the air.
In all cases, these research groups are focusing on SARS-CoV-2, but also thinking more broadly about other respiratory viruses, including those lying in wait to potentially usher in the next pandemic.
Quick, cheap, easy, PCR-like results
The foundation for UCSB’s SARS-CoV-2 test is the highly sensitive technology called loop-mediated isothermal amplification (LAMP), which has long held promise as an alternative to PCR. The UCSB group had already successfully used it to detect the large bacterial load that signifies a urinary tract infection [1], but LAMP didn’t work for the far smaller clinical threshold of an RNA viral infection. The issue was the so-called primer-dimer problem that erroneously amplified the virus count and therefore yielded false positives.
Rather than viewing the problem as insurmountable and moving on, the UCSB team saw it as a challenge. “We decided there was no turning back until we figured it out,” said research leader Michael J. Mahan, Ph.D., UCSB professor of molecular, cellular, and developmental biology. “It took us almost a year and a half, seven days a week, and some 500 tries, but through old-school biochemistry, including deconstructing the reaction conditions, and manipulating of the pH, salt, temperature, concentration of enzyme, and concentration of sampled saliva all at the same time, we finally felt we had solved the primer-dimer problem.”
The first tests of their new LAMP system had ideal results: It identified SARS-CoV-2 as well as two strains of influenza at a very high sensitivity rivaling that of PCR, and accomplished the feat in just 25 minutes compared to the hours required for PCR results. “That’s when we knew it wasn’t a one-and-done technology, and we could use it for many things, including home-based testing for COVID,” Mahan remarked (Figures 1 and 2). They published their work in JAMA Network Open [2].
Figure 1. After 18 months of intense work, a research group at the UCSB developed a new cell phone app and lab kit that transformed a smartphone into a rapid and accurate COVID detection device that can be readily adapted for other pathogens. (Image courtesy of Ryan Allen and Peter Allen, Second Bay Studios.)
Figure 2. UCSB group found a way to use LAMP technology to detect infection with even very low levels of the SARS-CoV-2 virus. Compared to PCR, their smartphone-based real-time LAMP (smaRT-LAMP) technology is at least as sensitive, and is much less expensive, and far faster. Pictured (left to right) are group leader Michael Mahan, Ph.D., and group members Scott Mahan, Douglas Heithoff, and Lucien Barnes. (Photo courtesy of UCSB.)
They quickly put together a reusable kit that is a platform for home use (Figures 3 and 4). “All the components are super inexpensive: some LED lights, a hot plate, a cardboard box and something to hold your sample,” Mahan said. “And it’s very simple to use: You spit in a cup, pour it into the reaction mix that we developed (about $7 per test), put it on a hot plate, and turn it on and connect your smartphone. Then, 25 minutes later, your phone shows either a green dot that tells you that you’re uninfected, or a red dot that not only tells you that you’re infected, but also quantitates it.” The quantification could be used to determine if an individual is highly infectious, and with tests over time, determine how well a COVID patient is responding to treatment.
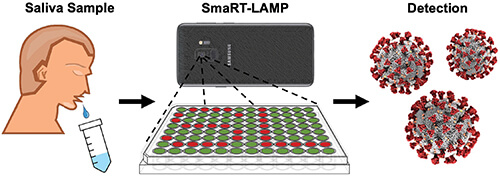
Figure 3. User’s role in the smaRT-LAMP technology is minimal: deposit a saliva sample on a tray, add reaction mix to the sample, turn on a hot plate beneath the tray, and set a smartphone on top. The technology does the rest, alerting the user in 25 minutes if it detected any virus, and how much. (Image courtesy of UCSB.)
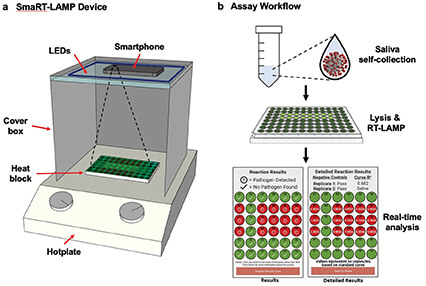
Figure 4. At left, the SmaRT-LAMP device includes LEDs that are affixed to the inside top of a cardboard box, which covers a heat block on a hotplate. The smartphone camera is directed toward the samples. At right, the smaRT-LAMP reaction mix is loaded onto a heat block, which initiates amplification. The smartphone app displays the results as a red circle if pathogen is detected, or green if none is found. (Image courtesy of UCSB.)
“Comparing apples to apples, a PCR test costs $100–150, and ours costs $7,” he said. Rapid antigen tests can also be quite inexpensive, but while a negative result with PCR or their smartphone-based real-time LAMP (smaRT-LAMP) signifies a person is not infected, a negative result with a rapid antigen test doesn’t rule out an infection [3]. Mahan remarked, “I think there should be better education about what rapid tests are really designed for: if-you’re-positive-you’re-infected, but if your negative, it does not necessarily indicate you are not infected. They’re just not sensitive enough for that, and that has led to outbreaks in work, school, and family settings.”
The smaRT-LAMP technology for virus detection is a game changer, Mahan asserted. “It’s a new dawn for molecular diagnostics: It’s as—or more—sensitive than PCR; it’s faster and cheaper than PCR; it’s isothermal so you don’t need fancy equipment; and it’s easy to use. In other words, it is a good platform for home-based testing, and also for bringing state-of-the-art diagnostics to resource-limited settings.”
Mahan is confident other researchers will use the technology to detect additional pathogens, including emerging viruses. “We didn’t patent anything, and everything is freely available to all right now, including the smartphone app (http://bacticount.com/). In fact, in the first week following our JAMA Network Open article, there were more than a thousand downloads of the app,” he said. “What we want to do is something that is good for the world, and we thought open sourcing it was the best way to do that.”
Early-detection app
The Scripps approach draws on the stream of data already being collected by Fitbits and other popular wearable fitness trackers. Prior to the advent of COVID-19, the group had already begun exploring whether aggregated data on resting heart rate (RHR) and sleep levels forecast the real-time ebb and flow of seasonal influenza across geographic regions [4], and found “a strong correlation in changes in wearable signals prior to outbreaks of influenza-like illnesses,” said Giorgio Quer, Ph.D., director of artificial intelligence at Scripps, and distinguished lecturer for the IEEE Communications Society (Figure 5). “So, when COVID-19 started, we saw an opportunity to help fight this new virus.”
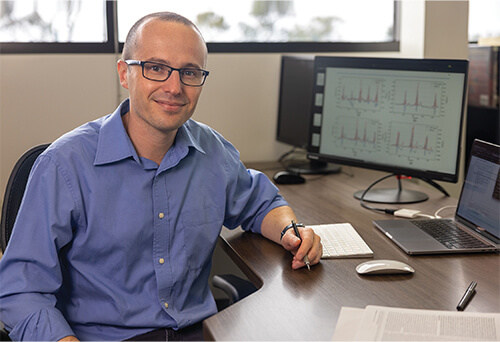
Figure 5. Giorgio Quer, Ph.D., at the Scripps Research Translational Institute, and his research group developed a machine-learning algorithm that uses fitness-tracker data collected through the DETECT study to predict COVID-19 infection. They found that changes in RHR, sleep, and activity are evident even before patients experience symptoms, and also appear among asymptomatic patients. (Photo courtesy of Giorgio Quer.)
In late March 2020, the researchers launched a study they called DETECT. Participants downloaded the MyDataHelps research app (https://detect.scripps.edu/) and consented to share data from fitness trackers, and also report flu-like symptoms and any COVID-19 test results (Figure 6). Initial findings of data from some 30,500 participants showed that subtle changes in an individual’s tracker data appeared early in the course of infection with SARS-CoV-2, and could “significantly improve the distinction between symptomatic individuals who were positive or negative for COVID-19 beyond symptoms alone” [5].
Figure 6. Scripps researchers hope to continue using the data to investigate what fitness-tracker data can reveal about post-COVID effects and COVID vaccines. (Image courtesy of Scripps Research.)
The researchers followed up by designing a machine-learning algorithm to dive deeper into the tracker data, especially RHR, and found that slight elevations in an individual’s RHR—typically by about 2 beats per minute—occurred with infection, and appeared in both presymptomatic and asymptomatic individuals [6]. “So, we can tell the user that they are at high risk for infection, and it might be important to be tested for COVID,” Quer said.
Besides identifying presymptom and asymptomatic infection, continued use of the DETECT app may also provide valuable information about post-COVID effects [7] and COVID vaccines [8], Quer said. For instance, the researchers have found that for some individuals baseline heart rate remains slightly elevated for 80 days after being infected with COVID-19, and a subset of patients show a higher elevation of up to 5 beats per minute. That lingering elevation signals that the patient is not fully recovered, he noted, “so potentially we can alert the participant and make them aware that they need to take care of this even after a follow-up test is negative.”
In addition, the researchers have noted that most individuals experience some slight rise in RHR in the day or two after receiving the vaccine. For those who do not show a rise in RHR, he said, this could suggest “a lowered immune response and possibly indicate the need for an earlier booster.”
Quer and his group are also hoping to begin a broader prospective study to demonstrate the clinical value of the app: Was an alert to a participant effective in spurring them to take a COVID test, or to avoid visiting relatives or going in to work? Can the app ultimately help slow the spread of the virus within a community? He remarked, “This is why it’s so important to bring together the engineering world and the clinical world, so we can understand how we can work together to put this algorithm and app into practice and not only help in the long term with COVID-19, but with any potential viral illnesses that come along in the future.”
Exposure-detecting air sampler
Before COVID-19, Yale researchers had developed an air-sampling wristband and clip as a way to measure personal exposure to air pollutants [9]. Aerosolized chemicals passively deposited onto a polymer film (polydimethylsiloxane, or PDMS, surface) within the device, and the researchers then used mass spectrometry to identify the type and amount of chemicals present.
“Very early on in the development of these chemical air samples, my collaborators and I recognized that respiratory viruses also had an airborne component: people expel and spread them through coughing and breathing, So we were already headed in that direction with our research, but then COVID came along and really accelerated that work,” said research leader Krystal Pollitt, Ph.D., assistant professor of environmental health sciences, and chemical and environmental engineering at Yale (Figure 7).

Figure 7. Krystal Pollitt, Ph.D., is leading a research project that has developed a small, lightweight, wearable clip that can be used to determine exposure to SARS-CoV-2. (Photo courtesy of Yale School of Public Health.)
The Yale group came up with a small, lightweight air sampler, called the Fresh Air Clip, that easily attaches to a lapel or collar. It incorporated the same type of PDMS film for passive collection of SARS-CoV-2 [10]. To analyze the captured virus, however, the researchers had to switch tactics. “We modified the shape of the sampler to allow for enhanced uptake of aerosols and droplets, including making the perforations larger and adding the Yale “Y” logo to the front so it prevents fingers from getting in, but still has a very effective opening surface area for collecting airborne material,” Pollitt said. “And because we were dealing with fairly low airborne concentrations (of virus), we spent quite a bit of time refining the extraction protocol to make sure that we were maximizing the recovery efficiency.”
Using a droplet digital PCR approach, the researchers were able to detect the virus with high sensitivity, and also ascertain exposure levels. Although the Fresh Air Clip currently is a research tool, Pollitt envisions it having application for individuals, such as teachers, health care workers, or restaurant servers, who would wear the clip to determine personal exposure risk, so they know when they should test for infection or quarantine. It could also be placed in a room to assess area viral levels, and signal whether spaces were low-risk, or high-risk and potentially in need of administrative or other infection-control measures, she said. Such area sampling could be particularly useful as people are returning to office and other public spaces. She remarked, “This would help them feel comfortable and give them reassurance that their risk of COVID infection is low.”
The researchers are continuing their work to expand the air samplers to identify other respiratory viruses, including seasonal flu bugs, but are putting most of their focus on scaling up the Fresh Air Clip and potentially commercializing it. “It’s great to have it as a research tool, but getting it to areas of need and making the best use of the technology is really what we’re hoping for.”
References
- L. Barnes et al., “Smartphone-based pathogen diagnosis in urinary sepsis patients,” EBioMedicine, vol. 36, pp. 73–82, Oct. 2018. Accessed: Mar. 3, 2022. [Online]. Available: https://www.thelancet.com/action/showPdf?pii=S2352-3964%2818%2930356-6
- D. M. Heithoff et al., “Assessment of a smartphone-based loop-mediated isothermal amplification assay for detection of SARS-CoV-2 and influenza viruses,” JAMA Netw. Open, vol. 5, no. 1, Jan. 2022, Art. no. e2145669. Accessed: Mar. 3, 2022. [Online]. Available: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2788464
- U.S. Centers for Disease Control and Prevention. (Feb. 22, 2022). Test for Infection. Accessed: Mar. 3, 2022. [Online]. Available: https://www.cdc.gov/coronavirus/2019-ncov/testing/diagnostic-testing.html
- J. M. Radin et al., “Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: A population-based study,” Lancet Digit. Health, vol. 2, no. 2, pp. e85–e93, Feb. 2020.
- G. Quer et al., “Wearable sensor data and self-reported symptoms for COVID-19 detection,” Nature Med., vol. 27, no. 1, pp. 73–77, Jan. 2021.
- M. Gadaleta et al., “Passive detection of COVID-19 with wearable sensors and explainable machine learning algorithms,” npj Digit. Med., vol. 4, no. 1, p. 166, Dec. 2021.
- J. M. Radin et al., “Assessment of prolonged physiological and behavioral changes associated with COVID-19 infection,” JAMA Netw. Open, vol. 4, no. 7, Jul. 2021, Art. no. e2115959.
- G. Quer et al., “Inter-individual variation in objective measure of reactogenicity following COVID-19 vaccination via smartwatches and fitness bands,” npj Digit. Med., vol. 5, no. 1, pp. 1–9, Dec. 2022.
- E. Z. Lin et al., “The fresh air wristband: A wearable air pollutant sampler,” Environ. Sci. Technol. Lett., vol. 7, no. 5, pp. 308–314, Jan. 2020.
- D. M. Angel et al., “Development and application of a polydimethylsiloxane-based passive air sampler to assess personal exposure to SARS-CoV-2,” Environ. Sci. Technol. Lett., vol. 9, no. 2, pp. 153–159, Jan. 2022.