The production of antibodies for therapeutic use in clinical medicine has become a focus of the biotechnology industry. In 2021, four of the top six selling prescription drugs were monoclonal antibodies, leading to a reported revenue of over US $67 billion dollars. Thus, it is no surprise that the otherwise arcane rules around antibody patents have become increasingly important to drug companies, health care providers, and consumers alike.
To understand antibody patents, one must first understand the basics of antibodies. Our body’s immune system needs a mechanism to identify and neutralize foreign and internal threats. Antibodies are the proteins that accomplish this task. As antibodies circulate throughout the circulatory and lymphatic systems, they react to antigens, which are most commonly foreign substances such as bacteria and viruses. An antibody works by binding to a specific region on an antigen called the epitope. After the antibody binds, other components of the immune system can then identify the bound substance as a threat and respond accordingly.
An antibody consists of four chains—two heavy and two light—that form a Y-shape. At the ends of the Y are complementarity-determining regions (CDRs), which determine how the antibody interacts with antigens. The combination of CDRs is a highly variable component that differentiates antibodies. This variability results in a vast number of possible types of antibodies. As a result, different antibodies may bind to different epitopes on the same antigen, and different antibodies may even bind to the same epitope using different mechanisms.
The understanding of antibody structure and purpose has changed over time. In turn, the process of patenting antibodies has shifted alongside the advancing technology itself. Antibody patents used to contain broad claims focused on function. These claims defined how the antibody interacts with an antigen—what it did or was supposed to do. These older patents included little description of the antibody itself. But broad patent claims covering antibodies’ function or application are now being invalidated with more regularity by the U.S. Patent and Trademark Office (USPTO) or in subsequent litigation. In response, patent lawyers have been drafting antibody patents to be more specific by including a detailed explanation of the antibody’s structure by describing the specific combination of CDRs and heavy and light chains. This narrowing of scope of antibody patents could have important consequences for drug pricing, public health, and innovation.
Evidence
Evidence of the shift in antibody patents can be observed by examining antibody patent descriptions. Figure 1 shows that over time, the percentage of patents that define the antibody according to its antigen (a function-based description) has fallen dramatically, reaching 0% by 2019. By contrast, the percentage of patents that define an antibody by its structure reached 100% in the same year.
The shift in antibody patent description from function to structure has occurred because of two requirements in the Patent Act, the federal legislation that defines the criteria for earning a patent in the USA. Section 112(a) of the statute requires that the inventor’s written description and drawings be sufficiently complete to enable a “person having ordinary skill in the art” to make and use the invention without having to engage in an undue amount of experimentation. In other words, patentees must clearly explain what their invention is (the written description requirement), as well as how to make and use it (the enablement requirement). These requirements ensure that, in exchange for receiving a 20-year federal right to exclude others from the invention via a patent, the inventor gives a meaningful disclosure of the invention into the public domain, allowing other inventors to learn from the discovery and potentially develop improvements. This is the process by which patents are supposed to contribute to the advancement of scientific and technical knowledge.
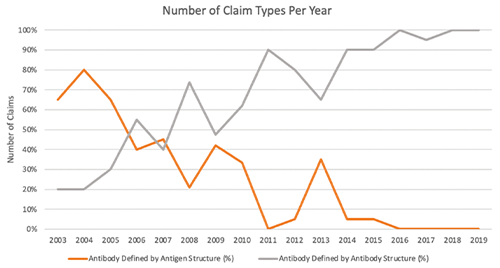
Figure 1. Variation in claim types of antibody in patents issued by USPTO, 2003–2019. [Image courtesy of Berkeley Technology Law Journal (forthcoming 2023).]
Almost all current antibody patents contain detailed descriptions of specific antibody structure to avoid being rejected due to enablement and written description requirements. This shift in the characteristics of antibody patents mirror the evolving ways in which antibodies are used.
In the previous century, monoclonal antibodies were applied primarily to research and diagnostic purposes rather than to treat disease. It therefore makes sense that most antibody patents were defined by the antigen and not based on a disclosure of the antibody structure. Accordingly, during this time period, it made sense to allow patents to broad antibodies without regard to the epitope of the target antigen.
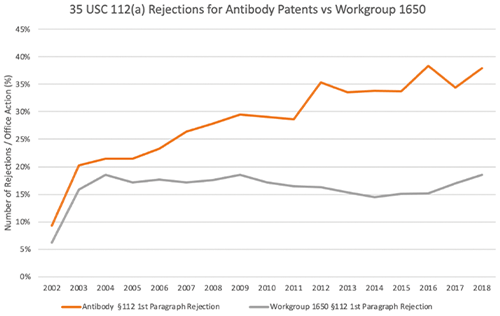
Figure 2. 112(a) rejections for antibody patents versus workgroup 1650 patents, 2002–2018. [Image courtesy of Berkeley Technology Law Journal (forthcoming 2023).]
In the past few decades, monoclonal antibodies have been increasingly developed for the treatment of diseases. When used as therapeutic agents in humans, even the slightest changes in the structure of an antibody could produce different patient outcomes. Accordingly, we have now moved into a period when antibodies can no longer be defined solely based on their antigen-binding partner, but require a detailed description of the antibody structure.
For example, adalimumab (Humira), a monoclonal antibody that also happens to be the world’s top revenue-generating brand-name drug, acts by binding with specificity to tumor necrosis factor-alpha (TNF-alpha) to inhibit its interaction with the p55 and p75 cell surface TNF receptors. Humira uses this property in treating a number of inflammatory conditions. The value of adalimumab lies in the fact that the antibody binds to a specific epitope of TNF-alpha. Adalimumab’s known clinical effects comes from its ability to target TNF-alpha and not bind or inactivate TNF-beta (also called lymphotoxin), which may have different effects on the body [1]. Thus, specific structural elements of the antibody itself are essential for the antibody’s activity as a therapeutic.
An example of a narrow claim in a patent covering this technology would be “An isolated human anti-TNFa antibody comprising the amino acid sequence of <description of the relevant antibody light chain region> and a heavy chain variable region comprising the amino acid sequence of <description of the relevant antibody heavy chain region>, wherein the antibody comprises an IgG1 heavy chain constant region and a kappa light chain constant region” [2]. This claim would cover only the specific antibody that was described by its specific light and heavy chain regions. Thus, unlike the previous broad functional antibody claim in the hCG example, current antibody patents are defined by claims to their narrow specific antibody structures.
The evolution of antibody patents has led to new controversies related to patents and their effect on innovation in this field. On the one hand, patent claims that are too broad stifle innovation, allowing individual entities to shut down competitors by gaining sweeping claims to large amounts of intellectual property. On the other hand, if claims are too narrow, then the patents become less useful in blocking competition and private investment in the high initial research costs leading to antibody discovery may be dampened.
Potential solutions
Patent law attempts to incentivize innovation by providing exclusive rights over an invention for a limited period of time. Currently, we may not be optimizing innovation in antibody-based drugs because we are not providing broader rights over antibody technologies. Patents are meant to be a quid-pro-quo exchange: patentees disclose the invention into public domain in exchange for a federal right to monopolize the invention for a limited amount of time. If patents only protect the narrowest of antibodies, and if it is too easy for competitors to design around these patents, then drug companies simply may not invest in creating these new drugs due to the uncertainty of return on their investment.
If one is concerned that the pendulum has swung too far to the side of narrow antibody structure patent claims, one possible solution could lie in using functional claiming combined with a legal tool called the Doctrine of Equivalents (DOE). Functional claiming allows patentees to claim the function of their invention and the specific means of accomplishing that function. Since such a patent only covers the invention’s specific mechanism of accomplishing its function, the DOE extends that coverage to all other mechanisms deemed equivalent. But if another method is found to do the same task, its invention would not infringe on the initial patent.
With functional claiming combined with DOE, antibody patentees would be given more freedom to apply for claims that are broader than just the specific antibody structure, since the new patent would cover the antibody and its functional equivalents. Other antibodies that bind to the same antigen but act in different ways or through different means would be left uncovered and available for other inventors. The difficulty with this solution, however, lies in determining which antibodies are truly equivalent to those outlined in a patent.
Another potential solution can be found in an even more arcane legal tool called the “Reverse Doctrine of Equivalents” (reverse DOE). Reverse DOE is a rarely used mechanism in which inventions are allowed to infringe on other patented inventions as long as they do so in a substantially different way. For example, if a new invention accomplishes the same function as described in the claims of another patent but uses a completely different mechanism, then reverse DOE would allow the new invention to escape patent liability. Accordingly, new antibodies that have completely different mechanisms of action could escape patent liability, even if they would directly infringe a broader pioneer patent.
Applying the reverse DOE in the antibody patent situation may help foster innovation because courts would receive more leeway to approve broader, function-based antibody claims as part of the initial antibody patent. After granting broader claims, courts could then use the reverse DOE to approve patents on new antibodies that accomplish the same function and bind to the same antigen as already-approved products, but do so through a different mechanism of action. Thus, reverse DOE allows us to eat our cake and have it too. Pioneer inventors would be able to obtain broader patent rights reflecting the greater initial investment and greater risk of failure. Additionally, other inventors could obtain narrower patents for similar antibodies that are truly distinct and innovative, and ideally are more effective or safer for patients.
In the rapidly growing antibody market, it is important to implement optimal patent policy to foster innovation. Patent examiners and courts have increasingly used the enablement and written description section 112(a) rejections to invalidate broad, functional antibody claims. Firms have reacted by changing their patent claiming strategy. Most approved antibody patents today contain only narrow claims based on a detailed description of antibody structure. Granting only the narrowest of patents, however, may not be optimal for future investment in innovation. Using functional claiming combined with DOE or applying the reverse DOE would allow broader claims for antibody patents and support the creation of new antibody drugs as well as allow sufficient room for fair competition. This would be a step in the right direction for developing antibody based drugs that are safer, more effective, and able to treat more diseases while keeping costs down for patients.
Acknowledgment
The work of Theodore W. Teng was supported by the Roxbury Latin School O’Connell Grant. The work of Aaron S. Kesselheim and S. Sean Tu was supported in part by Commonwealth Fund and in part by Arnold Ventures.
References
- N. H. Ruddle, “Tumor necrosis factor (TNF-α) and lymphotoxin (TNF-β),” Current Opinions Immunol., vol. 4, pp. 327–332, Jun. 1992.
- J. G. Salfeld et al., “Human antibodies that bind human TNF-alpha,” U.S. Patent 8 372 401, Feb. 12, 2013.



