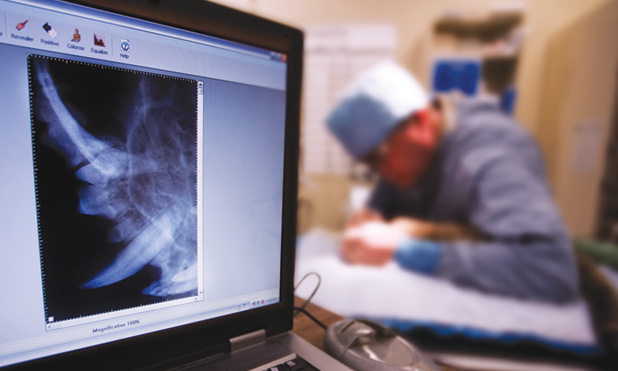
Dogs have bad breath. But when Montana sheep rancher Katy Harjes noticed her collie, Hoshi, had particularly bad breath and facial swelling, she was concerned that the symptoms might be a sign of something serious. She was right; ten-year-old Hoshi had squamous cell carcinoma, a common type of oral tumor found in dogs. The cancer had not metastasized, but the damage was extensive enough that part of Hoshi’s lower jaw needed to be removed. Luckily, Hoshi was a suitable candidate for a state- of-the-art bone regrowth procedure developed by Frank Verstraete, B.V.Sc, Dr.Med.Vet., M.Med.Vet., and Boaz Arzi, D.V.M., oral surgeons at the University of California (UC), Davis, School of Veterinary Medicine. Consequently, Katy and Hoshi embarked on a 15-hour road trip to California.
At UC Davis, biomedical engineers used computed tomography (CT) scans to create a three-dimensional (3-D) model of Hoshi’s skull, which Verstraete and Arzi used to contour and fit a titanium plate that would form the basis for Hoshi’s replacement jaw. This 3-D printing of the model skull before surgery cut down on Hoshi’s time in the operating room and under anesthesia.
In surgery, the titanium plate was screwed into the healthy part of Hoshi’s lower jaw. In this plate, the surgeons inserted a sponge-like scaffold material that had been soaked in bone morphogenetic protein, or BMP, which stimulates the growth of new bone cells. With this technique, new bone starts growing within two weeks. Within ten weeks, the new bone had integrated with the existing bone (Figure 1). Hoshi’s return visit six months after surgery showed that she had significant bone regrowth, and Katy reported that Hoshi was returning to her normal life.
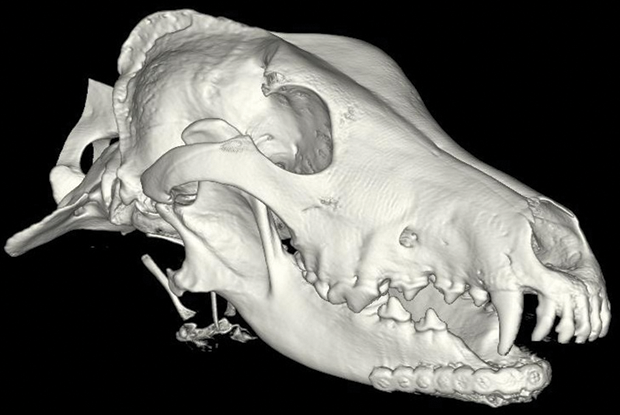
Verstraete and Arzi have performed jawbone regrowth procedures on 32 dogs from across the country. Some dogs such as Hoshi had cancer; others had severe dental disease or traumatic injuries. Thus far, all the procedures have been a success. “We worked out the best combination of the plate, the scaffold, and the BMP at the right concentration so that we now have very, very predictable results,” says Arzi. “Basically, we can guarantee that the jaw will be regrown.”
Verstraete and Arzi recently collaborated with orthopedic surgeons on a study that used BMP to help heal fractures in limb bones in dogs [ 1], and they continue to develop techniques for different oral surgery applications, including tackling upper jaw reconstruction. “The upper jaw has a complicating factor in the sense that there’s the nasal cavity, which is a contaminated environment,” says Arzi. “We’re not there yet, but we’ll get there eventually.”
This pioneering bone regrowth procedure is just one example of the many new techniques and technologies being developed by top veterinary schools as they increasingly rely on principles from the field of biomedical engineering. These advances are on the cutting edge not only of veterinary medicine but also of biomedical engineering. This means that while these veterinary schools are improving the lives of countless animals, they may also be laying the groundwork for novel improvements to human health.
Healing Hearts
An estimated 10% of dogs develop a serious heart condition called mitral valve regurgitation as they age. This condition is particularly common in aging small-breed dogs such as Chihuahuas, cocker spaniels, dachshunds, and Pomeranians.
Normally, the mitral valve separates the left ventricle and left atrium, preventing blood from going the wrong way back into the atrium. In a dog with mitral valve regurgitation, the two leaflets of the mitral valve have become thickened and rigid and thus cannot completely close, resulting in blood being leaked or “regurgitated” into the atrium.
As the condition worsens and the regurgitation becomes more severe, the heart becomes less efficient. The dog’s body then attempts to compensate by producing more blood, causing fluid to accumulate in the dog’s lungs. Eventually, this fluid buildup can lead to congestive heart failure and death.
The beginnings of the condition are largely invisible. Symptoms generally include common issues such as shortness of breath, chest pain, and dizziness, so an owner of a dog with mitral valve regurgitation may think that the dog is simply getting older and slowing down. Unfortunately, the only treatments for dogs are medicines that address the symptoms of the disease but not the leak itself.
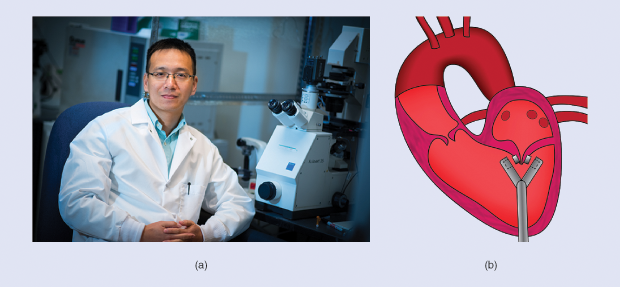
The leak can, however, be repaired with open-heart surgery, an invasive procedure that is prohibitively expensive for all but the wealthiest dog owners. Ke Cheng, Ph.D., associate professor of regenerative medicine at the North Carolina State University (NCSU) College of Veterinary Medicine and the University of North Carolina (UNC)-NCSU Joint Biomedical Engineering De- partment, wants to change that [Figure 2(a)]. He is developing a device for mitral regurgitation that he hopes will be more accessible to dog owners—and less invasive. “We do still have to cut down the chest, but we don’t have to open the heart,” says Chang.
His group has created prototypes of a novel device they call MitraMag that uses magnets to close the leak. MitraMag has a clamp, which a surgeon inserts through the chest and then positions over the two leaflets of the mitral valve [Figure 2(b)]. Each side of the clamp contains a magnet. Using ultrasound imaging, the surgeon can be certain that the clamp is positioned perfectly to close the valves. Once it is in place, the surgeon pulls a trigger to fire the magnets, sticking the leaflets together and closing the leak. The clamp is then retracted, but the magnets remain in place. Cheng hopes that the magnets become embedded within the valve cells, ensuring that they don’t move from where they belong.
Cheng and his colleagues are about to begin testing the device on pigs to be certain of safety. Then they plan to do a trial in dog patients. If the device proves successful in dogs, it may eventually be used in humans (more than four million people in the United States have significant mitral valve regurgitation). Cheng is even considering a future biodegradable device. “Something that you could do to not leave anything in the patient’s body. That’s perfect,” he says. “That’s always the number one choice.”
Training Surgeons

Before a surgeon can learn how to regrow a jaw or insert a device such as MitraMag, he or she first needs to learn the basics—such as how to suture. For years, veterinary students have practiced their suturing techniques on banana peels, orange peels, and the foam padding that goes under carpeting. These are not ideal substitutes. “The thing that frustrated me was that they just didn’t have a normal feel,” explains Dean Hendrickson, D.V.M., professor of surgery at Colorado State University’s College of Veterinary Medicine and Biomedical Sciences. This was the impetus that led Hendrickson to cofound a startup company called SurgiReal, which produces lifelike surgical models for veterinary and medical students (Figure 3).
SurgiReal products include simple suture models and models of dog legs and horse jugular veins that students can use to practice drawing blood or giving intramuscular injections. “We have models that simulate horses, dogs, and cats,” says Hendrickson. “We even have some that simulate rodents and rabbits.” They also produce models of hollow organs such as the stomach, intestine, and bladder, and they are working on developing models of other organs. In addition, they have almost completed a prototype of a canine model that contains sensors so that students can get feedback about their palpation skills.
The products are made from multiple varieties of silicon. Hendrickson and his team measured the thickness and compressibility of actual animal tissues to make their models as lifelike as possible. This means that SurgiReal can create custom models, such as those with more subcutaneous fat.
As of now, over 200 programs—including veterinary, medical, nursing, and physician assistant programs—are using SurgiReal products, and Hendrickson remarks that students have been enthusiastic about the models. “The nonbleeding ones they like because they can use wherever: at home, at their kitchen table, at the library, wherever,” says Hendrickson. “You can make an incision, suture it, take the sutures out, redo that at least ten times in each incision, and so they get a chance to put in a lot of suture and practice.”
Hendrickson says that the use of SurgiReal models is decreasing the number of cadavers used in veterinary training. A recent study out of Iowa State University found that using a SurgiReal model of horse intestine was just as effective as using cadaver intestine for teaching students how to use a particular suture pattern [2]. Hendrickson hopes it will also reduce practice using live animals: “I think we’re gaining ground on that as people see the success and the value of using the models.”
Curing Chronic Problem Wounds
Chronic problem wounds—wounds that won’t heal as they should—pose challenges in both veterinary and human medicine. For Jonathan McAnulty, D.V.M., Ph.D., who is professor and chair in the Department of Surgical Sciences at the University of Wisconsin (UW) School of Veterinary Medicine, finding a way to treat these wounds required a change in perspective. Instead of finding another medication that could be drizzled on the wound, he thought about changing the “nonfertile soil” of the wound bed so the cells that were trying to heal the wound could better do their jobs.

With this approach, McAnulty developed Microlyte Ag, an antimicrobial wound dressing for use in animals and humans (Figure 4). The basis of the Microlyte dressing is a polymeric membrane loaded with silver nanoparticles [3]. The polymers are charged molecules that adhere very tightly to the cells on the wound bed. This tight localization at the cell surface is what’s transformative about this dressing. It allows silver, an active antibacterial agent, to be held right where the bacteria that can prevent a wound from healing like to hang out. Silver kills over 99% of bacteria—including drug-resistant strains such as MRSA—and certain fungi.
The Microlyte Ag dressing uses much less silver than other silver dressings do. This is key because using too much silver kills not only the bacteria but also the cells that are trying to heal the wound. “Cells are trying to grow in there and heal that wound, and if you’re killing those cells with your treatment, it just holds everything back,” says McAnulty.
While the dressing can be used on any wound or incision site, Microlyte Ag may be particularly useful for chronic wounds. McAnulty, who is a small-animal general surgeon, once treated a cat who was caught in a window sash, dangling there for close to five hours. The cat had a serious fracture that was treated with surgery, but this didn’t end the cat’s struggles. “The wound started healing but then just stalled. It basically stayed the same size for five months,” explains McAnulty. “When we started treating that with the Microlyte, after three or four days we could measure that it was already smaller, and then after about three weeks it was healed.”
Other potential applications of Microlyte Ag, recently approved for use in humans, include treating burns, coating mesh implants used to reconstruct parts of the body, application on exposed bone plates following orthopedic repairs, and application within incision sites near easily contaminated tissues.
McAnulty sees this as a platform technology. He’s tested dressings that have gallium instead of silver, which can be used as antibiofilm agent [4]. “The advantage of that is when bacteria are in a biofilm, they’re in a kind of a very privileged environment, and they’re very hard to kill no matter what you hit them with,” says McAnulty. If you can get them to return to a free-swimming state, they’re much easier to kill with silver or other antibiotics. “We’re also looking at putting in analgesics [to the wound dressing] so that when you use it to treat something like burns, for instance, which have a lot of pain associated with them, we can get a multimodal dressing that can impact the infection as well as the discomfort of the patients,” he continues.
Speeding Up Diagnostics
Diagnosing illness and injury in animals can be tricky; they can’t tell anyone how they’re feeling, so early symptoms can go unnoticed. Unfortunately, this means that serious problems can go undetected until it’s too late. Consequently, a new generation of biosensor diagnostic devices has been developed that use enzymes to detect signs of brain injury, liver damage, and even cancer.
Roy Cohen, Ph.D., a research scientist at Cornell University’s College of Veterinary Medicine who is working on developing these sensors, got inspiration for them from an unlikely place: mammalian sperm. “We call it biomimicry,” says Cohen. In most body cells, glycolytic enzymes are free floating, but in sperm they are tethered to the cytoskeleton, allowing the sperm to be much more efficient in producing energy from small amounts of glucose.
Cohen and his group have developed a platform technology that uses tethered enzymes to detect biomarkers of various diseases. The technology works like this. A drop of blood or other sample is inserted into a cartridge, where it comes into contact with a bioengineered enzyme mixture (Figure 5). If a biomarker for that particular disease or condition is present, it interacts with the enzymes and produces a luminescent readout—all within a matter of minutes.
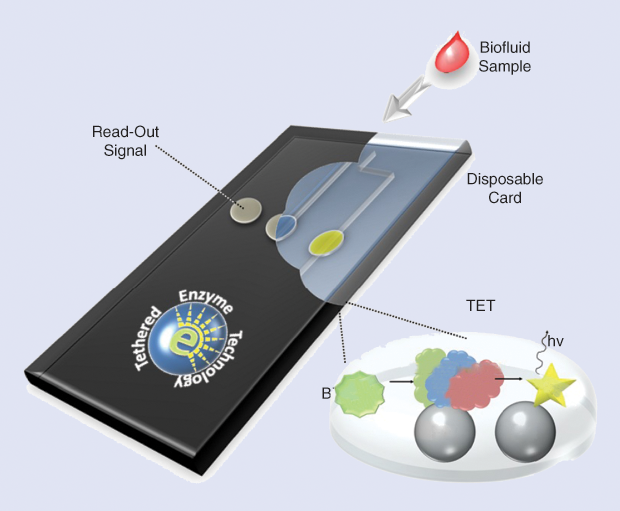
There are several advantages of this tethered enzyme technology over currently available techniques: it is fast, it requires a very small sample volume (ten microliters), and it is very sensitive. “It’s at least as sensitive as antibody–antigen reactions, and it’s much faster,” says Cohen.
As a platform technology, these biosensors have the potential to detect any type of disease or injury that has a biomarker. Last year, Cohen’s group published a study showing that a sensor could be used to detect a biomarker for brain injuries called neuron-specific enolase [5]. A biosensor such as this may one day be used to test for concussions on the playing field or detect signs of stroke in patients before they even get to the hospital.
According to Cohen, another advantage of using this type of diagnostic test, rather than one that depends on antibody–antigen interactions, such as the commonly used ELISAs, is that it is not a species-specific system. This means the same biosensor could be used to detect cancer biomarkers in humans, dogs, cats, or other species.
Right now, the group is developing a technology that can rapidly detect drug-induced liver damage in dogs and cats. Cohen hopes to have the sensor out for preliminary testing within the next year. He is also tackling hemangiosarcoma, a difficult-to-detect cancer common to golden retrievers and boxers. The goal, he says, is “a device in every vet clinic.” That way, “just another drop of blood goes into the sensor and gives you a heads up if anything seems suspicious.”
These devices are still in the design stage—Cohen is collaborating with other scientists to house the biosensors in disposable microfluidic cards that could be read in a handheld instrument. Meanwhile, the list of potential applications keeps growing and growing. “We’re super excited about this,” says Cohen. “Every other week we get new ideas for new diseases.”
References
- A. M. Massie, A. S. Kapatkin, M. C. Fuller, F. J. Verstraete, and B. Arzi, “Outcome of nonunion fractures in dogs treated with fixation, compression resistant matrix, and recombinant human bone morphogenetic protein-2,” Vet. Comp. Orthop. Traumatol., vol. 30, no. 2, pp. 153–159, Jan. 2017 doi: 10.3415/VCOT-16-05-0082.
- S. S. Caston, J. A. Schleining, J. A. Danielson, K. D. Kersh, and E. L. Reinertson, “Efficacy of teaching the gambee suture pattern using simulated small intestine versus cadaveric small intestine,” Vet. Surg., vol. 45, no. 8, pp. 1019–1024, Nov. 2016. doi: 10.1111/vsu.12554.
- M. Herron, A. Agarwal, P. R. Kierski, D. F. Calderon, L. B. Teixeira, M. J. Schurr, C. J. Murphy, C. J. Czuprynski, J. F. McAnulty, and N. L. Abbott, “Reduction in wound bioburden using a silverloaded dissolvable microfilm construct,” Adv. Healthcare Mater., vol. 3, no. 6, pp. 916–928, June 2014. doi: 10.1002/adhm.201300537.
- M. Herron, M. J. Schurr, C. J. Murphy, J. F. McAnulty, C. J. Czuprynski, and N. L. Abbott, “Gallium-loaded dissolvable microfilm constructs that provide sustained release of Ga(3+) for management of biofilms,” Adv. Healthcare Mater., vol. 4, no. 18, pp. 2849– 2859, Dec. 2015. doi: 10.1002/adhm.201500599.
- R. Cohen, J. P. Lata, Y. Lee, J. C. Hernández, N. Nishimura, C. B. Schaffer, C. Mukai, J. L. Nelson, S. A. Brangman, Y. Agrawal, and A. J. Travis, “Use of tethered enzymes as a platform technology for rapid analyte detection,” PLoS One, vol. 10, no. 11, pp. e0142326, Nov. 2015. doi: 10.1371/journal.pone.0142326.