If ever an industry was in need of both incremental and disruptive innovation, it is today’s health care industry. Realizing the full potential of innovation across the spectrum of health care environments is critical to address the well-documented, emerging global crisis generated by the aging of the population, the obligation to increase access for all to the best standard of care, and the societal imperative to contain costs. In addition, as budgets at funders such as the U.S. National Institutes of Health (NIH), the U.K. National Health Service (NHS), and others are increasingly constrained, it is more important than ever to increase the efficiency and effectiveness with which investments in fundamental R&D translate into products, services, and procedures that improve the health and well-being of people around the world.
The painful conundrum and related opportunity are these: why has health care, one of the most technology-intensive industries, with tens of billions of dollars invested in R&D annually in the United States alone, not only failed to see more of an impact from that investment but also lagged behind the innovation performance of other industries? The 15+ years of experience at the Consortia for Improving Medicine with Innovation and Technology (CIMIT) in Boston, Massachusetts, offers insight into this puzzle and evidence that solutions are available to help realize the full power of technology and innovation in health care.
Examples abound of the power of technology and innovation to enable disruptive step changes in performance while simultaneously slashing costs. One of the best known is Moore’s law in semiconductors, which projects a doubling of central processing unit capacity every 18–24 months, typifying the exponential power of technology. Innovator and futurist Ray Kurzweil extended Moore’s law to show that whenever a specific technology approaches some kind of physical limit, a new technology platform emerges to allow the exponential growth to continue, bypassing the perceived barrier.
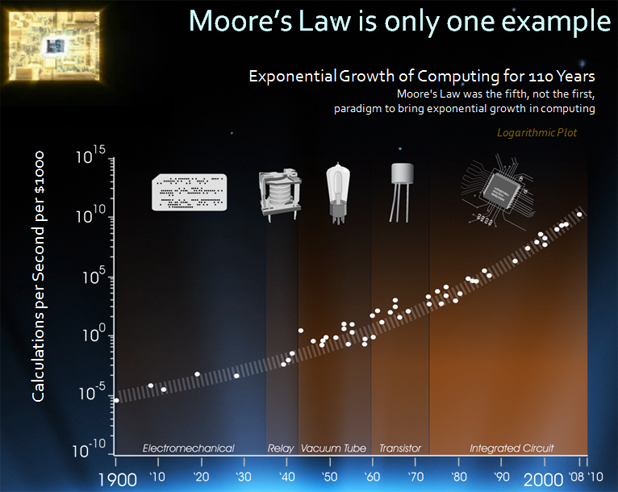
What, then, is the basis for the general perception, if not reality, that new technologies and innovations in health care mostly result, at best, in incremental improvements at higher costs? Counter examples exist, such as the dramatic cost savings in genomic sequencing, outpacing Moore’s law, and showing what technology can do in a laboratory setting, but the actual impact on health and wellness remains elusive. Experience in the pharma industry, codified as Eroom’s Law, illustrates the disappointing results in practice. Rather than show an improvement over time, the trend for new drug approvals by the U.S. Food and Drug Administration (FDA) per inflation-adjusted U.S. dollars spent on R&D shows a decrease of 50% every nine years—a negative rather than positive exponential growth.
Further, while no generally accepted law exists for health care as a whole, the U.S. macroeconomic picture of health care is consistent with Eroom’s law. For example, as described by Michael Mandel, chief economic strategist at the Progressive Policy Institute, employment in the health care sector is rising faster than the growth of the population. The increase in employment, including from physicians, nurses, paraprofessionals, and support staff, far outstrips what can be explained by the increase in older Americans, and this is a key reason for rising healthcare costs—employment costs account for a much larger fraction than the cost of new drugs, supplies, and capital expenses for new technology. The most likely conclusion is that productivity in health care is actually in decline, with technology not improving productivity as it has in other industries.
There are many barriers unique to health care cited as reasons for the inability of technology to transform the health care industry. These include misaligned incentives, technological complexity, organizational fragmentation, physician training and bias, documentation burdens, and regulatory convolutions. While there are unique challenges in every industry, it is hard to argue that health care is so unique and different from all other industries that the impact of technologies is fundamentally different. CIMIT’s premise is that lessons from other industries can inform the way that technologies and innovations can be better developed and used in health care.
CIMIT and the Health Care Innovation Cycle
CIMIT was founded in 1998 as a “living lab” to study and catalyze health care innovation. A “center without walls,” its charter is to foster multidisciplinary and multi-institutional collaborations that bridge silos of medicine, technology, and business to rapidly improve patient care. CIMIT was an innovation itself: a new type of public–private partnership designed to support innovative translational research by leveraging and combining expertise from across academia, industry, and government to address unmet medical needs. By way of example, much of CIMIT’s early funding came from the U.S. Department of Defense (DoD) through its Telemedicine and Advanced Technology and Research Command (TATRC) to help address the rapidly changing health care needs of soldiers and their families, with dual-use potential for the civilian population.
The CIMIT founders and leadership over the years recognized that addressing major challenges in translational research required treating the process of innovation in health care as a discipline itself. They had learned from experience that researchers working alone could not meet the transformative improvement needed in diagnostics and treatments for complex diseases and medical conditions. Instead, it requires close collaboration among innovative clinicians, engineers, scientists, and implementation experts. CIMIT created and cultivated an innovative model as a resource for translational research teams. Pioneered in Boston, CIMIT has since been widely emulated in other locales, successfully connecting clinical, engineering, and commercialization communities across academic departments and institutions together with patient advocates, funders, and companies.
CIMIT is a voluntary consortium of independent institutions and represents a massive network of experts in medicine, life sciences, physical sciences, information technology, regulatory practice, and commercialization with a shared passion to bring about paradigm-changing outcomes. Steadily growing since 1998, the CIMIT consortium now draws on 13 academic medical centers, universities, and laboratories in the greater Boston area along with four international affiliates; the international affiliates include Manchester: Integrating Medicine and Innovative Technology (MIMIT) in the United Kingdom the Agency for Science, Technology and Research (A*STAR) in Singapore and the Eastern Health Alliance (EHA) in Singapore and just recently the AIAQS for the Catalan Health System in Spain.
[accordion title=”CIMIT Consortium Members”]
Consortium Institutions
- Beth Israel Deaconess Medical Center
- Boston Medical Center
- Boston University
- Brigham & Women’s Hospital*
- Children’s Hospital Boston
- Draper Laboratory*
- Harvard Medical School
- Massachusetts General Hospital*
- Massachusetts Institute of Technology*
- Newton-Wellesley Hospital
- Northeastern University
- Partners HealthCare
- VA Boston Healthcare System
* Founding Members
International Affiliates
- MIMIT (Manchester, United Kingdom)
- A*STAR (Singapore)
- Eastern Health Alliance (Singapore)
- AIAQS (Barcelona, Spain)
[/accordion]
CIMIT provides funders and investigators alike with a single portal into a huge pool of clinical and technology domain experts with an enormous diversity of talent and extensive expertise in areas relevant to all aspects of health care. Many of these experts are well-networked, international thought leaders whose contributions to innovative approaches to patient care have become standards of care.
From the start, CIMIT recognized that sophisticated innovation methods along with technologies developed for nonmedical uses could be applied to unmet medical needs. In addition, CIMIT observed that early stage, multidisciplinary translational projects have little chance of funding from conventional sources. In response, CIMIT focused on the often unrecognized and undervalued function of actively facilitating collaborations between multifunctional and multidisciplinary teams throughout the project life cycle to increase the potential for significant near-term clinical impact.
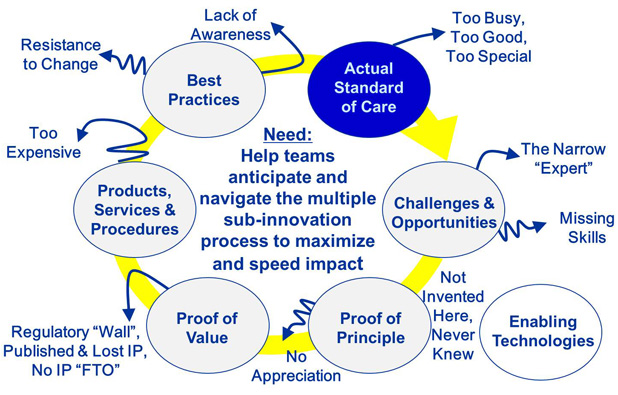
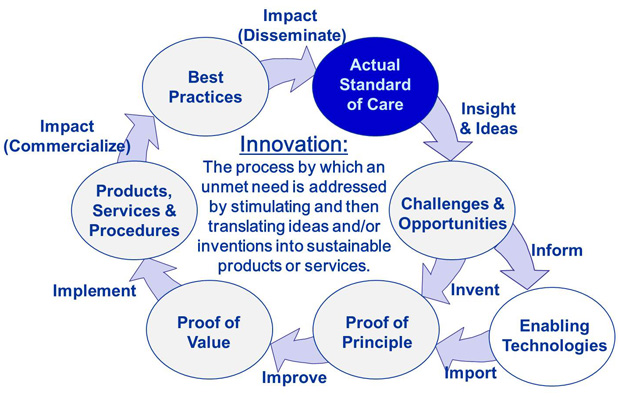
Over the years, CIMIT has evolved processes to efficiently and effectively define an important unmet medical need—perhaps the most critical of all ingredients—and then stimulate and support ideas to the point of creating clinical impact. We call the set of processes encountered on this journey the health care innovation cycle. CIMIT’s experience results from facilitating more than 600 projects through some portion of or the entire journey. In working closely with project teams, CIMIT’s facilitators focus on anticipating and then addressing the many reasons that disruptions occur in the transition from one stage of the health care innovation cycle to the next or a later stage.
CIMIT Leadership and Governance: Top-Down Support for the CIMIT Mission and Model
CIMIT’s leadership team works closely with CIMIT faculty and investigators and enjoys significant bottom-up support from its investigators as a result. In addition, it receives important top-down support from its Executive Committee: the chief executive officers/presidents of all CIMIT consortium institutions. They provide critical infrastructure support, but more importantly, by participating personally, they demonstrate the organization’s commitment to translating technologies into improved patient care.
Great efficiencies result from having a formal legal agreement between CIMIT and each consortium institution and affiliate. This establishes explicit expectations so that collaborative projects can start fast and with minimal administrative burden. For example, to overcome the challenges of managing intellectual property (IP) in multi-institutional projects, and to expedite IP filings by institutional tech transfer offices, the agreement sets clear guidelines that ensure each institution retains the rights to all IP generated by its investigators. The agreement also provides a process by which the institutions collaborate in prosecuting a patent, sharing the costs and returns. CIMIT is a neutral broker and retains no rights to investigator-generated IP, even though it provides seed funding and facilitation.
The CIMIT Model: Integrated Methods and Processes to Innovate in Health Care
Over the years, CIMIT has developed various integrated methods and processes referred to collectively as the CIMIT model to find, fund, and facilitate collaborations that impact patient care.
- Finding significant unmet medical needs and identifying potential collaborators to work on solutions occurs through CIMIT-sponsored meetings, forums, innovation congresses, and other physical and virtual social networking opportunities. These events bring together diverse stakeholders from academia, MedTech, biopharma, the venture community, medical foundations, and representatives from government agencies, including the U.S. DoD, NIH, FDA, and Defense Advanced Research Projects Agency. By proactively engaging the broad CIMIT community to discover, evaluate, and address unmet needs, these events stimulate novel ideas and spawn new collaborations, leveraging synergies from across different technical and clinical domains, and encouraging creative problem solving.
- Funding investigator teams has taken many forms at CIMIT:
- Innovation Grants support early stage, high-risk, collaborative research for improving patient care, with an emphasis on devices, procedures, diagnosis, or systems of care. They are typically one-year projects with budgets up to approximately US$100,000, intended to scientifically derisk a novel idea by showing early stage proof of concept.
- Accelerator awards support the facilitation and execution costs of projects that have the potential for a commercial handoff in 12–18 months. Whereas Innovation Grant projects are proof of principle and scientifically derisk a project, projects that receive an Accelerator Award start with a proof of value and then develop and implement a plan to derisk projects technically and commercially for hand-off to industry via the lead institution Tech Transfer Office in 18–24 months.
- Fostering “rising stars” takes on many forms at CIMIT. CIMIT sponsors several awards, fellowships, and student projects to attract the brightest new minds into this community of innovation. For example, the CIMIT Young Clinician Award recognizes outstanding clinicians within the consortium, early in their careers, who are engaged in the development of transformative innovations in health care. The awards are an important way CIMIT helps retain the best and brightest minds by providing a viable career-advancement path while encouraging the pursuit of high-risk–high-payoff translational work.
- Facilitating the formation and progress of teams of clinicians, engineers, and commercialization experts to propose and conduct translational research is the charge of CIMIT faculty, including site miners, program leaders, and accelerator executives, as well as CIMIT facilitators:
- Site miners are senior members of the clinical research community who literally mine their institutions for projects and investigators deserving of funding and coaching. Site miners open and maintain dialogues between the clinicians and researchers at the front lines of health care and technology within their institutions. They work with CIMIT and its program leaders to find and assess areas of clinical unmet need, seek out and connect clinicians and scientists who have creative ideas about applying technologies to solve these patient-care challenges, provide seasoned guidance and mentorship for early career investigators, and serve as expert reviewers of proposals submitted to CIMIT in its grant processes. Site miners are the glue to connect people and ideas across the cultural walls of consortium institutions and even across the boundaries separating the departments within them. Collectively, CIMIT site miners have thousands of interactions with both established and potential investigators annually.
- Program leaders are responsible for CIMIT’s clinical and technical focus areas. They are senior researchers, faculty at a consortium institution, who seek out and bring together innovative scientists, clinicians, and engineers across the consortium to solve major unmet medical needs within their particular programmatic area. As national and international authorities on one or more medical or engineering specialties, program leaders serve as head coaches for the teams of investigators within their program area, imparting advice and offering encouragement as these researchers plan and execute their collaborative projects. They challenge CIMIT to pursue emerging areas of clinical need where devices and other technologies could make a powerful difference in the standard of care.
- Accelerator executives are successful serial MedTech entrepreneurs and business leaders who proactively engage with teams to accelerate a handoff to industry within 12–18 months. This relatively short time requires that the accelerator executives work as a team as well as working intimately with the project team to not only advance the technology but also to develop and begin executing a complete strategy for moving the solution into practice, specifically transitioning the project from the academic to a commercial setting.
- CIMIT facilitators are CIMIT staff members who provide facilitation and support for investigators. The expertise ranges from contracts and compliance to human use approvals and proposal writing.
CoLab: CIMIT in the Cloud to Encourage, Manage, and Measure Innovation
Effectively traversing the health care innovation cycle involves navigating numerous interrelated processes with geographically dispersed people and groups operating behind numerous institutional firewalls throughout the CIMIT consortium and beyond. In response to the resulting logistical challenges and to support other process-related functions, CIMIT leadership launched the development of a suite of cloud-based software tools—CIMIT CoLab, a health care collaboration platform—to manage those processes efficiently and to facilitate communications and collaborations across disciplines, functions, and institutional walls.
CoLab’s developmental direction is established with the leading institutions across the globe with whom CIMIT is collaborating. CoLab currently comprises configurable modules that map to the health care innovation cycle. As an integrated solution, it can work alone or in combination with an institution’s existing infrastructure to augment available social media, knowledge, and innovation management tools. It combines four key elements in one platform:
- communities: individuals and groups linked in dynamically defined roles
- processes: user-configurable, codified workflows for collaborative practices, such as the CIMIT model
- portfolios: projects and activities organized for reporting and management oversight
- content: secure, structured, Web-accessible information such as documents, wikis, videos, links, ratings, decisions, etc.
CoLab is being used by CIMIT and its collaborators to enable effective collaborations in managing processes, such as proposal and “challenge” calls; working together in secure, virtual workspaces; capturing metrics as well as providing a real-time dashboard on the status of a portfolio of projects, ideas, or initiatives. CIMIT alone was able to increase the number of ideas in its annual call by 50% while using less than one-half the staff time.
CIMIT Clinical Impact Study: Proof the CIMIT Model Works to Drive Solutions to Patient Care
By treating innovation in health care as a process, the pathway to improvement starts with measurement. As such, CIMIT conducted a clinical impact study (CIS) in 2009 and updated it again at the end of 2012 (final report in preparation) to assess the outcomes of projects on which CIMIT had applied its “find, fund, and facilitate” model. Each analysis was limited to projects initiated more than three years before the analysis occurred to give projects a reasonable time to generate results. For both studies, the CIMIT leadership, with the help of investigators and program leaders, captured, quantified, and analyzed the impact created by the CIMIT-funded projects, including the resulting products, procedures, and services. The team quantified input metrics, such as funding and facilitation, as well as output metrics, such as patients impacted, publications, patents, and career impact.
The 2012 CIS evaluated a subset of CIMIT’s entire portfolio that consisted of 538 Innovation Grant and Accelerator projects, representing US$62 million in funding. This portfolio comprised 175 stand-alone projects and 363 projects conducted as part of 106 packs [defined as a group of tightly interconnected projects, typically under multiple principal investigators (PIs), for which the clinical impact created could not be attributed to any single project]. As of 2013, the projects CIMIT has supported have resulted in more than:
- 36 companies or new product lines being formed
- 460-issued US patents (with foreign counterparts in addition) along with more than 320 patent applications pending
- 700 peer-reviewed publications
- A 12:1 ratio in follow-on funding generated:
- 3:1 for funding directly to the PI
- 9:1 for commercial investment.
Correlations between the input and output variables provided insights that CIMIT is using to further improve how it uses resources. Some examples of the key lessons learned that emerged from studying the trends and correlations include the following:
- Sweet spot: CIMIT’s greatest “bang for the buck” occurred in supporting numerous high-risk, early stage innovative projects, with funding in the US$100,000–300,000 range. While more funding created more clinical impact, this range is where CIMIT spends its resources most efficiently, with other organizations providing follow-on funding to advance them further.
- Facilitation: Regardless of the size of the grant or the promise of the study, targeted and skilled facilitation is a powerful amplifier of success. In addition, while facilitation is valuable at any stage of the innovation cycle, from the preproposal phase onward, it is most valuable in the early stages, particularly including the prefunded team-formation stage.
- Packs: A closely related insight is that projects conducted in packs were much more likely to achieve commercial success than single projects. Even when normalized by the amount of CIMIT funding, packs received ~20 times more commercial funding and garnered almost three times more awarded patents (about nine patents for each US$1 million CIMIT invested in packs versus about three for individual projects per US$1 million CIMIT funding).
- Clustering: Projects and packs conducted as part of a cluster of activities are more effective than those done in isolation. Clusters represent thematic communities of interest, examples being: optical coherence tomography, simulation mannequins for training medics and first responders, and near infrared light for neurological and cancer treatment. Clusters are not managed by a single person or group—they are effectively facilitated by peers, CIMIT staff, and strong CIMIT program leaders. They benefit from the broader resource-rich environment of talent across the CIMIT consortium.
- New translational investigators: Junior faculty members were as successful as more senior investigators working within the CIMIT model to conduct translational research. Mentorship, raw talent, and “fresh eyes” were cited as potential equalizing factors along with the CIMIT facilitation.
We fully expect that the application of these lessons that have been learned over CIMIT’s 15+ years will create even better results in the future.
Lessons Learned in Other Locales
[accordion title=”The CIMIT Model in Singapore”]

A*STAR is the lead agency for fostering world-class scientific research and talent for a vibrant knowledge-based and innovation-driven Singapore (Figure S3). A*STAR oversees 14 biomedical and physical sciences and engineering research institutes, as well as six consortia and centers, located in and near the R&D complexes known as Biopolis (Figure S4) and Fusionopolis. A*STAR supports Singapore’s key economic clusters by providing intellectual, human, and industrial capital to its partners in industry. It also supports extramural research at the universities, hospitals, research centers, and with other local and international partners.
In 2009, the A*STAR MedTech Initiative was launched with the mission to promote R&D in the MedTech sector to foster a vibrant and sustainable ecosystem in Singapore. The A*STAR MedTech Initiative currently oversees three programs: 1) the Biomedical Engineering Program (BEP), 2) the A*STAR-CIMIT-Eastern Health Alliance (EHA) Collaboration, and 3) the Singapore-Stanford Biodesign (SSB) Program.
The BEP is the flagship program that seeks to fund and facilitate the development of MedTech innovation from idea to first-in-man. To do this, the BEP fosters collaborations between Singapore’s research performers and medical professionals to address unmet needs identified by the medical community. A competitive, multidisciplinary grant call is also administered yearly, in which all funded projects must be driven by needs identified by the clinical community and must demonstrate high potential for commercial outcome with a high likelihood to rapidly impact patient care.
Recognizing that the proven success of CIMIT in building a vibrant MedTech ecosystem in Boston could be leveraged to strengthen BEP, A*STAR has collaborated with CIMIT on growing MedTech innovations and activities between Singapore and Boston since January 2010. This mainly involves: 1) adopting and adapting CIMIT’s “find, fund, and facilitate” model that is aimed at creating a CIMIT-like consortium among research engineers at A*STAR and clinicians in local hospitals, 2) collaborating on shorter-term projects from a pipeline of clinically vetted ideas from CIMIT with a shorter timeline toward clinical applications, and 3) launching bilateral grant calls to initiate upstream and longer-term collaborative projects between Boston and Singapore engineers and/or clinicians to build capabilities, know-how, and IP in MedTech innovations.

Through the A*STAR-CIMIT Collaboration, A*STAR has successfully adopted CIMIT’s best practices by implementing the rigorous and highly selective process for finding, funding, and facilitating projects with high potential for improving care in the near term. The BEP currently engages the CIMIT faculty in the review of BEP project proposals. A*STAR has also adapted several initiatives, such as the BEP MedTech Innovation Forums, which are conducted quarterly, and the appointment of site miners in several of Singapore’s hospitals, universities, and A*STAR research institutes. A*STAR has also appointed its program leaders in the clinical domains of cardiology, ophthalmology, and neurology with the aim to build peaks of excellence within these areas. Two late-stage technology development projects have also been successfully brought into Singapore to increase the pipeline of commercially viable projects and adapt these technologies for the Asian market, one of which is now in clinical deployment. In November 2012, EHA joined as the third member of this collaboration. Accession to the collaboration allows EHA to leverage CIMIT’s expertise, as well as A*STAR’s research capabilities to introduce novel medical products, services, and procedures to EHA’s network of clinicians to improve patient care.
The innovations coming out of this collaboration are many. They include new technology to reduce hospital acquired infection through a high-tech hand-washing monitoring system, self-help kiosks to help improve patient care and alleviate long wait times in crowded clinics, a new technology to automatically screen and diagnose large populations for glaucoma, and a brain–computer interface system that can help patients get personalized stroke rehabilitation outside a rehabilitation center.

Also launched in January 2010, the SSB Program is a partnership between A*STAR, EDB, and Stanford University and is a talent development initiative that seeks to nurture and train the next generation of Asian medical device innovators in Singapore, serving Asia and beyond. Modeled after the established Biodesign Program at Stanford University, the SSB Program comprises the following components: 1) a one-year SSB fellowship, where fellows will undergo six months of training in the Biodesign process at Stanford University and will be based in Singapore for the remaining six months to bring their solution to the prototype or proof-of-concept stage; 2) the SSB Innovation Class, which allows graduate students at Singapore’s tertiary institutions to undertake a semester-long innovation class, providing students accelerated exposure to the MedTech innovation process; and 3) the SSB-organized seminars, where key opinion leaders around the world will share and discuss the latest industry insights and experiences on medical technology development.
[/accordion]
[accordion title=”MIMIT, the CIMIT Model Applied in Manchester”]
One of the first international affiliates of CIMIT was MIMIT.
Like CIMIT, the primary aim of MIMIT is to drive innovation to improve patient care. Clinicians, scientists, engineers, industry, tech transfer organizations, health economists, and investors are brought together in a structured way to catalyze development of innovative health care technologies through a rigorous analysis of clinical need and derisking of investment.

Led by Director Prof. Jackie Oldham, MIMIT forms a cornerstone of the Manchester Academic Health Science Centre (MAHSC), a partnership between The University of Manchester, Central Manchester University Hospitals NHS Foundation Trust, Manchester Mental Health and Social Care Trust, Salford Clinical Commissioning Group, Salford Royal NHS Foundation Trust, The Christie NHS Foundation Trust, and University Hospital of South Manchester NHS Foundation Trust.
During a four-year period, MIMIT has developed 38 projects identified by MIMIT site miners (Figure S2). These projects were selected from 148 unmet needs scoped from across the MAHSC partnership. Examples include repairing severed nerves, replacing damaged discs in the spine with novel microgels, next-generation colostomy bags, reducing ventilator-associated lung injury in children and adults, a new disposable tamponlike electrostimulation device to treat incontinence, and a device to help with swallowing post stroke.
MIMIT has provided £1.45 million initial direct investment (£15,000–100,000 per project) matched by an equal amount of indirect investment to support projects for approximately 12 months. To date, projects have received £5.1 million in direct funding to investigators (1:5 ratio) and £18 million in venture capital (VC) and industry investment (1:18 ratio). In addition, clinical research studentships/fellowships, and numerous publications/patents have arisen from MIMIT activity. Two of the MIMIT projects have won the Northern England Bionow Healthcare Project of the Year awards (2010 and 2012).

MIMIT projects have led to two new spinout companies, a third company has been taken up by a small to medium enterprise, and MIMIT is directly supporting two other small to medium enterprises in a joint project. Four projects are in commercial negotiations, and a further four are in the pipeline. In addition, MIMIT has contracted with three global companies to scope and validate unmet health care needs. A further seven projects have led to joint industry initiatives ranging from design to implementation.
MIMIT Statistics:
A summary of statistics is as follows:
- 148 unmet needs considered
- 38 projects developed
- £1.45 million MIMIT direct investment
- £5.1 million follow-on investment
- in excess of £18 million VC/industry investment
- two Bionow Healthcare Projects of the Year awards (2010 and 2012)
- more than 20 industry collaborations.
[/accordion]
The CIMIT model has been adapted and demonstrated to work just as effectively, if not even more so, in Manchester, United Kingdom, at MIMIT and in Singapore at A*STAR and the EHA (see “Case Study: MIMIT, the CIMIT Model Applied in Manchester” and “The CIMIT Model in Singapore”). A new collaboration in Catalonia is just starting. Success in adapting and implementing the CIMIT model in these locations has created more impact through the strong clinical and academic institutions that are in place. Through these experiences, we have learned that the model is not dependent on Boston’s uniquely rich ecosystem. We have found that some key lessons do exist in successfully adapting and using the CIMIT model:
- Institutional support and leverage: Top-down institutional support, engaged site miners (or equivalent), good alignment with institutional priorities, and active involvement of clinical and research staff as well as support from functional departments such as marketing, tech transfer, legal, etc., are critical. In addition, working closely with and heavily leveraging other local resources is equally important to focus the finite resources available. In Boston’s case, we are fortunate to leverage many organizations, including MassMEDIC, a trade organization of medical device companies, and the Mass Life Science Center, a state-funded initiative to grow the life science sector.
- Resources: A committed core team on the ground with a strong, well-connected leader and efficient methods to operate and connect people (with tools such as CoLab) along with a three- to five-year funding window to sustainability, most preferably from a number of sources to enhance sustainability.
- Metrics of success: Clear goals that are aligned with the priorities of the funders, be they oriented around general clinical impact (such as number of patients treated, cost savings, etc.), progress in treating specific conditions (such as trauma or neuropsych health), regional economic growth (number of jobs created, investment generated, etc.), or some combination, along with infrastructure to track the results.
CIMIT draws its power from networking the rich capabilities at its consortium institutions. As such, CIMIT has found that even more impact is created by networking CIMIT-like organizations across geographies. As the number of “nodes” in the network increases, thematic networks are being created, and solutions available in one locale are being used to address clinical problems in another.
Conclusions
Innovation in health care is an imperative. It is a process driven by clinical needs, and its practice should be studied, codified, shared, measured, and subject to continuous improvement. This is a well-accepted premise in most other industries, and CIMIT has shown it can apply in health care.
Successfully innovating to address an unmet clinical need does have unique challenges. It requires multidisciplinary teams of experts, often from different organizations, collaborating through a complex journey with misaligned incentives and strict constraints to protect patients from harm, operating in highly competitive and constantly evolving business and regulatory environments. Being able to navigate the journey through the cycle of innovation is a discipline itself. Historically, the discipline was “learned by doing,” often without training or mentorship, or even worse, with mentors that had learned lessons that no longer apply. The CIMIT model, at its most fundamental level, addresses these historical problems by treating innovation as a learnable and dynamic process, linking a network of collaborators, providing seed funding and a virtual support infrastructure, and delivering expert facilitation. The combination helps teams focus on addressing the right clinical issues, getting the right collaborators, streamlining administrative obligations, anticipating challenges, and making decisions throughout the journey to use scarce resources in a way that minimizes risk and maximizes patient impact. The CIMIT model works well in Boston and elsewhere when adapted. It also has the potential to create even more impact by linking hubs of medical innovation across the world to address important challenges. It offers funders, academic health care centers, universities, and the private sector a successful model from which to learn and build upon to realize the exponential power of technology and accelerate the health care innovation cycle.
Acknowledgments
The authors are grateful to all of the program leaders, site miners, investigators, executive committee members, and CIMIT facilitators whose work and shared dedication to patients and wounded warriors have made CIMIT possible. They also thank the DoD and TATRC for their unwavering support.



