Functional electrical stimulation is experiencing a renaissance, with new emphasis on therapeutic uses
Long a staple in physical therapy and rehabilitation clinics, functional electrical stimulation (FES) is experiencing a resurgence as it rides a wave of new technologies into new therapeutic uses. Mobilizing recalcitrant limbs and re-educating damaged nerves, FES is being deployed to assist with gait and balance, correct sleep apnea, and teach stroke patients how to swallow again.
At 71, Jan Grierson has had many experiences with assistive devices and surgeries meant to help her walk despite her lifelong struggle with cerebral palsy. As her mobility slowly degraded through the years, there have been night splints and ankle orthotics, and, starting more than 15 years ago, a walker that she nicknamed “the Harley.” She quietly loathed them all. But in March 2021, she became a “test pilot” for a San Francisco start-up’s new device. Wearing Cionic’s neural sleeve, which looks like a fabric legging, Grierson can now navigate hills, rutted roads, and dirt paths that have been out of bounds for nearly two decades.
“It peels back time and gives me mobility that I lost a long time ago,” she says. “Cionic helps me communicate with my legs. The neural sleeve is the only device in a lifetime of wearing devices that I actually look forward to putting on every day.”
Now, wearing the Cionic sleeve 10 hours per day, Grierson says, “I feel like I’m in the middle of a medical miracle” (Figure 1).

Figure 1. Wearing Cionic’s neural sleeve, Jan Grierson can hike on rugged trails with her husband Alan for the first time in 15 years. (Photo courtesy of Jake Kazakevich for Cionic.)
Improved tech creates new possibilities
It’s a miracle a long time in the making. The sleeve’s key technology, FES, first entered rehab clinics in the 1960s. Essentially, FES uses electrical current to cause a muscle contraction. Its primary medical use involves applying gentle electrical charges to muscles that have weakened or become paralyzed, replacing or enhancing the natural signal that should be coming from the brain.
Medical device makers began introducing FES orthotics about 50 years ago to stimulate a single leg muscle to aid walking in patients with upper motor neuron conditions such as cerebral palsy, multiple sclerosis, stroke, and spinal cord injuries who have difficulty lifting their foot as they walk, called drop foot. While the expensive and bulky devices helped some patients, they generally weren’t considered much better than static braces and manual physical therapy. But with the advent of microelectronics, wireless technologies, and machine learning algorithms, FES is only now experiencing a renaissance in a flood of new medical applications and research.
Two FES gait-correction devices remain prominent in the United States: the WalkAide system from Innovative Neurotronics and the L300 Go from Bioness, a subsidiary of Bioventus Rehab. The Odstock Dropped Foot Stimulator is widely used in the U.K. All three of these systems work by sending a low-level impulse to the peroneal, or fibular, nerve in the leg to signal the ankle dorsiflexors to lift the toes off the ground while walking.
Cionic’s neural sleeve, however, is the only one to address all four major muscle groups: hamstrings, quadriceps, calves, and shins. The sleeve’s 24 electrodes—divided between those groups—are sequenced by a machine-learning algorithm customized to meet the unique needs of each individual patient (Figure 2).
“Our innovation is to address a much wider variety of daily activities, including stairs, inclines and declines, and sitting/standing,” says company founder and CEO Jeremiah Robison (Figure 3), whose 13-year-old daughter Sofia was born with cerebral palsy.

Figure 2. Cionic’s neural sleeve connects to an app on the wearer’s smartphone to allow dynamic customization of its 24 electrodes to meet the unique needs of each patient. (Photo courtesy of Cionic.)
Applying his decades of experience working with embedded systems and machine learning for Apple, Openwave, Slides, and Jawbone, Robison first began developing the device in his garage in 2018, shortly after watching the crudely simple technology available to assess, but not treat, his daughter’s mobility. Before he sought funding, he said he wanted to be certain that this wasn’t just the dream of a frustrated parent. Now, after 50 prototypes and uncounted tours of research labs throughout the world, Cionic has begun a limited roll-out of its system. Potential users can register on the company’s wait list; the next release is scheduled for the second quarter of 2023.

Figure 3. Jeremiah Robison founded Cionic to develop technology that would help his daughter, who has cerebral palsy, enjoy more mobility. (Photo courtesy of Cionic.)
Robison believes a world of bionic wearable medical devices is inevitable. To support that, Cionic’s neural interface collects anonymized, granular movement data that with patient permission can be shared with researchers, physical therapists, and other health professionals studying movement and how FES can benefit others. Cionic also has developed open application programming interfaces (APIs) to make it easier for other researchers to develop their own FES systems [1].
“Ultimately, I would love for our technology to be something that permanently fixes gait and mobility differences, especially in degenerative diseases,” he says.
Moving to other parts of the body
Applications of FES don’t stop with walking, however. Bioness, which recently added an upper-leg cuff to work in concert with its calf-placed device, also has developed the H200 Wireless Hand rehabilitation system. Centered around a wrist-to-elbow brace and a handheld remote, the system uses custom-fit electrodes to deliver electrical pulses that can enhance grip and reduce muscle spasms from a stroke, spinal cord injury, or other central nervous system condition.
The device, which can be used in the home or in a rehabilitation center, coordinates activity between three groups of muscles: extensors, which open the hand; flexors, which allow the hand to grasp; and the thenar, which activates the thumb’s pinching movement. In addition to aiding hand function while using the device, some stroke patients also have seen long-term improvements that indicate FES is “re-educating” previously paralyzed muscles to accept signals from the brain [2].
“This kind of work is even more difficult in the forearm than in the leg, but the idea is very smart,” says Alberto Botter, who heads up the Laboratory for Engineering of the Neuromuscular System, or LISiN, at the Polytechnic University of Turin, Italy. “A huge issue of FES is the rapid development of muscle fatigue because the electrical stimulation triggers movement at a different frequency than the brain does. It triggers the larger motor units first, while the brain starts with the smaller motor units.”
To contend with that, Botter and his colleagues have been developing a way to interleave electrical stimulations between nerves and muscles to mimic more natural signals from the brain. Studying the effects with ultrasound, the team has determined that this interleaving (e.g., at 10 hertz on the muscle and then 10 hertz on the nerve) reduces the rapid fatigue that develops using muscle stimulation alone [3].
LISiN’s work also contributed to Cionic’s combination of both electromyography (EMG) and FES into a single electrode array, a challenging technical feat that allows the company to gather important movement data at the same time the neural sleeve is correcting a patient’s gait.
“With these electrodes embedded in textiles, the wearability, and the portability, and the possibility to use several channels of stimulation at once and to integrate them more easily with algorithms we can do so much more today than we could 15 or 20 years ago,” Botter says.
Sub-sensory signaling for proprioceptio
Others are reaching beyond today’s motor control with FES to introduce new forms of therapy. A team at the University of Delaware is working toward combining FES gait control with a sub-sensory form of FES called stochastic resonance to enhance posture and balance in children with cerebral palsy.
“The sensory side of the research has been long neglected,” says Samuel C. K. Lee, program director of the university’s Biomechanics and Movement Science Laboratory. Lee, who serves on the executive board of the International Functional Electrical Stimulation Society, has worked extensively over the last two decades with FES-equipped stationary tricycles and treadmills to increase muscle tone in young cerebral palsy patients [4].
Although his team had to develop their own equipment initially, FES stationary cycles now are commercially available and used to promote rehabilitation following spinal cord injuries. Now Lee’s research has moved toward what he calls “closing the loop” between motor control and proprioception.
“Postural transitions—sitting to standing, standing to walking, sitting down—challenge balance,” he says. “It’s only in the last few years that anyone has acknowledged that cerebral palsy also causes impairment in the sensory pathways. If we can combine what we’ve learned about motor control with the delivery of stochastic resonance stimulation, we believe we can retrain the nervous system for more normal sensory information return, vastly improving overall function.”
The combination of these two types of FES, he believes, will produce results far greater than the sum of the parts. “Even if we can’t train the brain with the improved sensation, we can gather the data we would need to develop a wearable device that could deliver the sensory stimulation that’s needed,” he says.
Branching out into sleep and swallowing
Other commercial devices are taking advantage of FES to help treat conditions as diverse as sleep apnea and dysphagia—difficulty swallowing often caused by a stroke.
To treat obstructive sleep apnea, a Medtronic spin-out called Inspire Medical Systems delivers short electrical pulses to the hypoglossal nerve, prompting a sleeping patient’s tongue to move out of the airway with each intake of breath. Unlike motor control FES systems, however, Inspire requires an implant. During an outpatient surgery, the FDA-approved device is positioned in a patient’s chest with a narrow catheter threaded up to just under the jawline. Before sleeping, the user pushes a button on a wireless remote to switch the device on. More than 24,000 patients across the United States and Europe have had the device implanted [5].
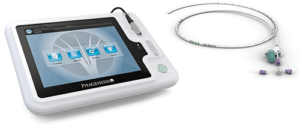
Figure 4. Phagenyx device induces a swallow reflex using an electrode at the back of the throat. The stimulation prompts reconnection of the neural pathway between the cerebral cortex and the pharyngeal nerve. (Photo courtesy of Phagenesis.)
Developed in the U.K. at the University of Manchester, the FES-based dysphagia treatment from Phagenesis is used broadly across Europe. In October 2022, the Phagenix system was granted FDA de novo approval as a “breakthrough device,” allowing it to enter the U.S. market (Figure 4). Used only in hospitals or rehabilitation centers, the system stimulates the pharyngeal nerve through an electrode-tipped nasal catheter placed at the back of the throat. Turned on for only 10 minutes each day for three days or six days, depending on the severity of the condition, the device prompts reconnection of the neural pathway between the cerebral cortex and the pharyngeal nerve. If used within 60 days of a stroke, the treatment usually improves or restores the patient’s ability to swallow [6].
“Essentially, it trains the brain how to swallow again,” says Anil Keni, the company’s vice president of marketing. “There is an emerging bolus of evidence that the earlier you treat, the better the long-term outcome is.”
The future of FES
The success of this kind of neuromodulation excites researchers who are eager to move FES beyond its muscle-control roots. Ken Yoshida, a founding member of the International Functional Electrical Stimulation Society and now a professor of BME at Indiana University–Purdue University Indianapolis, says he’s been thinking about neuromodulation since he was a kid watching Lee Majors play The Six Million Dollar Man.
“That man-machine interface really fascinated me. These were bionic prosthetics, but he was feeling things from those limbs. There had to be a neural interface,” he says. “Finally, I saw in the opening scene this tiny sign—‘neural interface.’” And then there was Luke Skywalker, who got a bionic prosthetic hand in Star Wars: The Empire Strikes Back, he said. “I’m in this because of science fiction.”
Yoshida now is researching a new waveform that appears primed to accomplish things a standard FES pulse waveform can’t do. And researchers have begun combining FES devices with brain–computer interfaces to create brain-controlled FES therapy systems. The next decade or so, he believes, will bring an exciting new wave of functional electrical stimulation devices.
“What can you do beyond controlling muscle? That’s the birth of neuromodulation,” he says. “I’m seeing papers showing that it’s helping people with spinal cord injuries to regain some function in their limbs, but nobody understands why it’s happening. The more we understand, the more we’ll be able to do.”
References
- J. Robison, R. Gibbons, D. Achelis, B. Bent, D. Wajda, and R. Webster, “Augmenting gait in a population exhibiting foot drop with adaptive functional electrical stimulation,” Jun. 2002. [Online]. Available: https://www.medrxiv.org/content/10.1101/2022.04.27.22273623v2.full
- I. A. Vozniuk, A. V. Polyakova, and D. V. Tokareva, “Neuroprosthetic technology bioness (exorobot) in the process of restoring motor and vegetative-trophic disorders in central paresis of the upper limb,” Bull. Rehabil. Med., vol. 5, no. 99, pp. 62–69, 2020, doi: 10.38025/2078-1962-2020-99-5-62-69.
- M. Carbonaro, O. Seynnes, N. A. Maffiuletti, C. Busso, M. A. Minetto, and A. Botter, “Architectural changes in superficial and deep compartments of the tibialis anterior during electrical stimulation over different sites,” IEEE Trans. Neural Syst. Rehabil. Eng., vol. 28, no. 11, pp. 2557–2565, Nov. 2020.
- A. Sansare et al., “Aerobic responses to FES-assisted and volitional cycling in children with cerebral palsy,” Sensors, vol. 21, no. 22, 2021, Art. no. 7590. [Online]. Available: https://www.mdpi.com/1424-8220/21/22/7590
- B. T. Woodson et al., “Upper airway stimulation for obstructive sleep apnea: 5-year outcomes,” Otolaryngology–Head Neck Surgery, vol. 159, no. 1, pp. 194–202, 2018. [Online]. Available: https://journals.sagepub.com/doi/abs/10.1177/0194599818762383?journalCode=otoj
- S. Hamdy, J. C. Rothwell, Q. Aziz, K. D. Singh, and D. G. Thompson, “Long-term reorganization of human motor cortex driven by short-term sensory stimulation,” Nature Neurosci., vol. 1, no. 1, pp. 64–68, May 1998. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/10195111/



