With an aging population, the incidence and prevalence of wound problems is on the rise. Bedsores (also known as pressure ulcers or decubitus ulcers) are painful, take months to heal, and, for many patients, never do, leading to other health problems. The condition has become so acute that treating bedsores is now a significant burden on the healthcare system. An estimated 2.5 million pressure ulcers are treated in U.S. hospitals each year, adding US$11 billion annually to health care costs.
Hospital-acquired pressure ulcers are among the most expensive hospital-acquired conditions, with treatment costs reaching up to US$130,000 per patient [1]. However, even while pressure ulcers are becoming a more common problem, paying for treatment is not getting easier. In 2007, the Centers for Medicare and Medicaid Services added hospital-acquired pressure ulcers to the list of hospital-acquired conditions for which treatment costs will not be reimbursed [1]. This leads to an increasing burden of costs on hospitals and patients.
How Pressure Ulcers Form
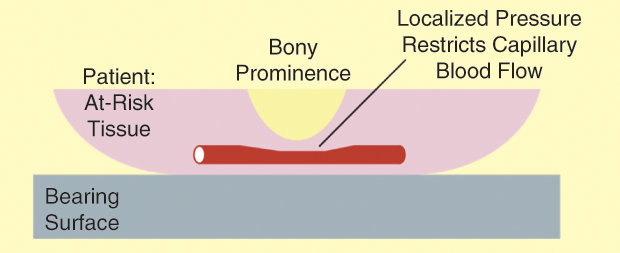
Figure 1 shows how pressure ulcers can form in at-risk areas of the body such as the heel, sacrum, scapula, ischium (sitting patient), or occiput (supine patient). When a sufficient fraction of a patient’s weight is supported in a region with a bony prominence, the resulting localized concentration of external pressure reduces the cross-sectional area of blood vessels, restricting blood flow and limiting oxygen supply to the at-risk tissue. If this external contact pressure is maintained long enough, the lack of oxygen leads to tissue death and the formation of pressure ulcers.
While many factors influence pressure ulcer formation, the main contributing factor is local contact pressure maintained over several hours, with additional contributions related to tissue temperature and relative humidity. When contact pressure exceeds approximately 30 mmHg, it impedes capillary blood flow, leading to decreased circulation and possible reperfusion injury. When this external contact pressure is maintained for a considerable period, it leads to a pressure injury. High pressure maintained for a short period of time, as well as low pressure for a long period, can cause soft tissue injury [2].
Who Gets Pressure Sores
Pressure ulcers tend to arise as a secondary ailment when patients are bedridden for treatment of some other illness or injury. This might be in a hospital or a nursing home. Patients particularly susceptible to pressure ulcers include those who are bedridden, immobile, neurologically impaired, malnourished, critically ill, and/or suffering from debilitating disease processes [2]. These patients’ sensory abilities are reduced, so they cannot feel the formation of a pressure ulcer. In a hospital setting, surgical patients who lie in a fixed position on the operating table for hours are also at risk. Studies have shown that pressure ulcers acquired in operating rooms have a more complex etiology than those in medical patients. This is due to circulatory and metabolic changes that occur with the use of anesthetic agents, leading to an increase in heat metabolism juxtaposed with the cool temperature in the operating room [3]. This occurs even in a setting involving military personnel: one Veteran’s Administration polytrauma rehabilitation center studied active duty admissions from 2003 to 2006 and found that 53% of casualty admissions from Operation Iraqi Freedom and 38% overall had pressure-related injuries (pressure ulcers, scarring unrelated to their field injuries, and so forth) on admission [4].
Pain and infection are known to complicate ulcers, increasing the length of a hospital stay. The presence of pressure ulcers is a poor prognosis factor for a hospitalized patient and, in general, represents a global decline in a patient’s health. The National Quality Forum currently recognizes 29 “never events”— adverse events that are unambiguous and serious but usually preventable. Any stage 3, stage 4, or unstageable pressure ulcers acquired after admission/presentation to a healthcare facility are included on this list.
For those patients who develop pressure ulcers, the consequences can be severe, including pain, depression, local infection and renal failure, infections to the bone (osteomyelitis), anemia, sepsis, gas gangrene, cardiovascular events, and even death. Unfortunately, health workers often cannot do the repositioning necessary to prevent pressure sores because of poor work environments, poor staffing levels, and lack of time.
The Limits of Current Preventable Measures for Pressure Ulcers
Prevention and management of pressure ulcers involve patient repositioning and pressure redistribution devices. The most commonly employed method of pressure ulcer prevention is pressure off-loading. This is usually achieved by caregivers physically turning bedbound patients every 2 hours. Some automated devices such as specialized beds and wheelchair cushions automate the offloading task. Turning patients regularly is a labor-intensive and time-consuming process. It places the caregivers at risk for workplace injuries and does not prevent all pressure ulcer incidents, even when strictly enforced.
Frequent repositioning of a bed-ridden patient may not be advisable if it results in reloading the same site too frequently [5]. In outpatient settings, wheelchair cushions are designed to off-load pressure from the ischial tuberosities and sacrum, but, unfortunately, they do not address other body parts. Certain devices also employ motion sensors that alert caregivers when a patient has not moved for a period of time. However, during transport, specialized beds are not practical, and motion monitoring may be entirely confounded by the movement of the transport vehicle. Sensors have also been used to map pressures on the bedding surface. This technology is not practical during transport, and the measured contact pressure is localized to a coordinate system on the bearing surface, not on the patient. Despite extensive work, there is no compact, affordable solution to preventing pressure ulcers in transport, hospitals, or home setting.
Our Vision for Treating Pressure Sores
Our long-term vision is to approach pressure ulcer prevention in an entirely different way: to develop a wireless, autonomously site-specific powered sensor system that would alert medical personnel to a potentially harmful level of contact pressure that suggests the possibility of a pressure ulcer. One or more sensor patches, placed on sites known to be at-risk areas on the patient’s skin, would monitor local contact pressure, temperature, relative humidity, and motion, communicating these measured parameters to a base station (or smartphone). Over time, machine-learning algorithms specific to various settings (e.g., aeromedical evacuation, hospitals, home care, and so forth) would provide an alert for immediate, direct intervention to prevent the formation of pressure ulcers. Our plan would improve patient care while easing the workload of caregivers. Use in operating rooms will also be beneficial to patients. During longer surgical procedures, it is inconvenient to reposition patients regularly. However, if an adequate warning is issued, it can be feasible for surgeons to reposition patients on an as-needed basis.
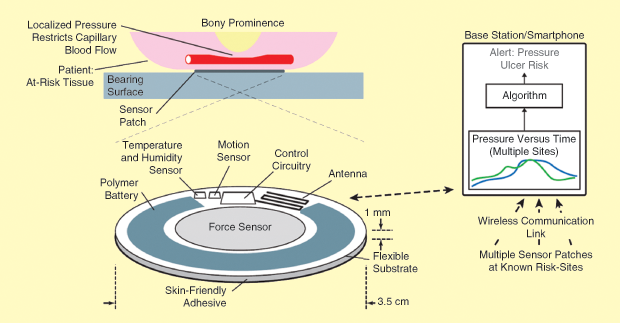
The desired attributes of our proposed approach are as follows:
- wireless communication
- the measurement of contact pressure localized to at-risk areas only
- a device that could work independently of caregiver or patient interpretation
- a patient-specific algorithm
- a device that could work for wheelchair-bound patients as well as those in bed
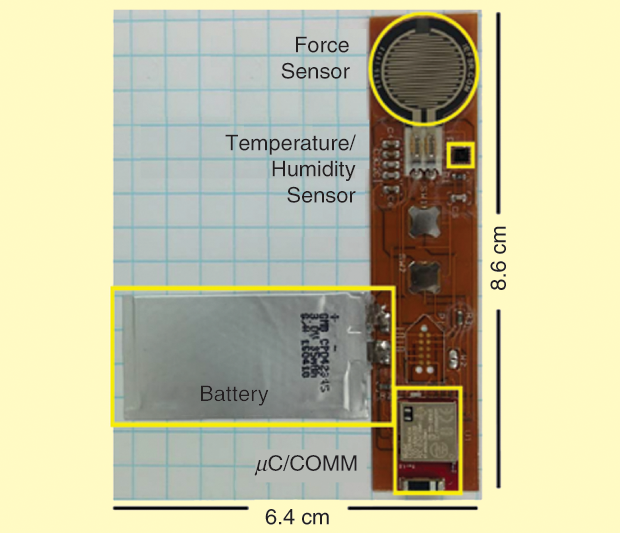
Our current implementation [6] includes a rectangular sensor patch consisting of a force sensor being used to measure pressure, a temperature and relative humidity sensor, and control circuitry capable of wireless communication via Bluetooth Low Energy. A polymer battery powers the system. Multiple patches can be configured to communicate with a single base station. The sensor patch has been designed for use in an animal trial. Although our long-term approach is to have the sensor patch in a circular shape with a diameter of 3.5 cm and a height profile of 1 mm, as seen in Figure 2, we chose a rectangular shape for our current implementation, as seen in Figure 3, to properly fit the dorsal sides of the Sprague-Dawley rats.
Our Experiment
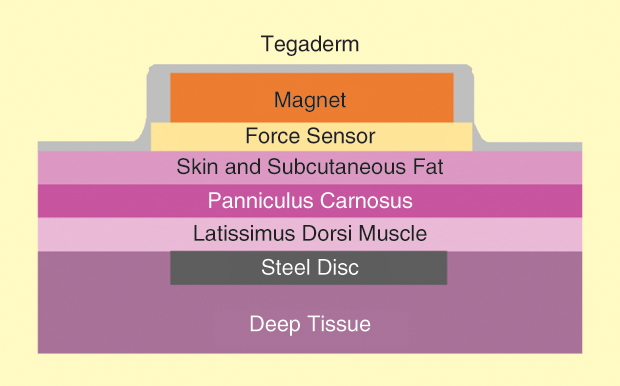
Steel discs were surgically implanted under the latissimus dorsi muscles of rats, as seen in Figure 4. Ten days after the operation, external pressure was applied to the skin, subcutaneous tissue, and musculature overlying the implant using magnets of varying strengths (30–150 mmHg). The sensor was placed between the skin and the magnet to collect data regarding pressure and time (1–8 h). Three days after pressure application, the animals were euthanized following the University of Massachusetts Medical School–Institutional Animal Care and Use Committee policy on humane euthanasia/recommended practices for rodent euthanasia. After euthanasia, the area of injury was graded clinically and histologically.
The actual pressures measured were routinely higher than the intended pressure. All rats who had the magnet application for more than 2 h had clinical evidence of pressure injury, and these were confirmed by histological analysis. At intended pressures of 120–150 mmHg, even for brief periods, there was injury to the muscular layer without corresponding epidermal or dermal injury.
Implementing machine-learning algorithms would enable drastic improvement in the prevention of pressure ulcers, more independence for mobile at-risk patients, and easing of caregivers’ workloads. The autonomously powered sensor system would alert a patient or caregiver to a potentially harmful level of contact pressure at a specific location that could produce a pressure ulcer. Ultimately, adoption of this technology would have tremendous impact for alleviating patient suffering and reducing health care costs. The high frequency of pressure ulcer occurrence, their adverse consequences for the patient and family members, and the massive costs involved in treatment justify enormous efforts to reduce the problem. Demographic trends will accelerate this demand as people live longer and have lower mobility later in life.
References
- W. V. Padula, R. D. Gibbons, R. J. Valuck, M. B. D. Makic, M. K. Mishra, P. J. Pronovost, and D. O. Meltzer, “Are evidence-based practices associated with effective prevention of hospital-acquired pressure ulcers in U.S. Academic Medical Centers?” Med. Care, vol. 54, no. 5, pp. 512–518, 2016.
- S. M. Quigley and M. A. Q. Curley, “Skin integrity in the pediatric population: Preventing and managing pressure ulcers,” J. Spec. Pediatr. Nurs., vol. 1, no. 1, pp. 7–18, Apr.–June 1996.
- B. Schouchoff, “Pressure ulcer development in the operating room,” Crit. Care Nurs. Q., vol. 25, no. 1, pp. 76–82, 2002.
- J. J. Harrow, S. L. Rashka, S. G. Fetzgerald, and A. L. Nelson, “Pressure ulcers and occipital alopecia in Operation Iraqi Freedom polytrauma casualties,” Mil. Med., vol. 173, no. 11, pp. 1068–1072, 2008.
- J. H. Meijer, P. H. Germs, H. Schneider, and M. W. Ribbe, “Susceptibility to decubitus ulcer formation,” Arch. Phys. Med. Rehabil., vol. 75, no. 3, pp. 318–323, 1994.
- D. Sen, J. McNeill, Y. Mendelson, M. D. Raymond Dunn, and M. D. Kelli Hickle, “Wireless sensor patch suitable for continuous monitoring of contact pressure in a clinical setting,” in Proc. IEEE NEWCAS Conf., June 2018, pp. 91–95.



