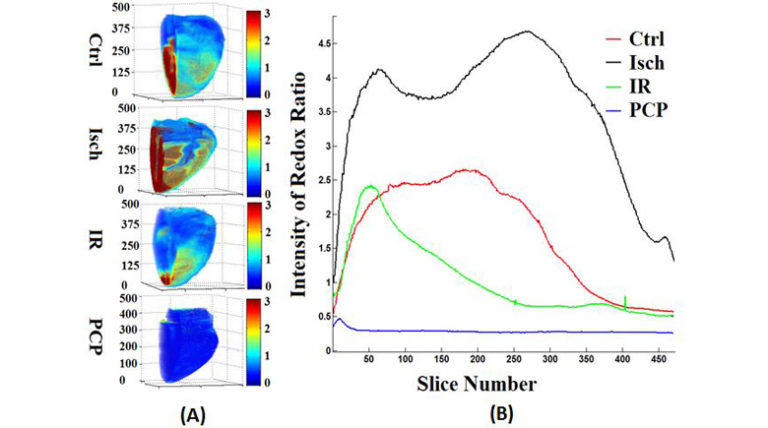
Objective: Oxidation of substrates to generate ATP in mitochondria is mediated by redox reactions of NADH and FADH2. Cardiac ischemia and reperfusion (IR) injury compromises mitochondrial oxidative phosphorylation. We hypothesize that IR alters the metabolic heterogeneity of mitochondrial redox state of the heart that is only evident in the 3-dimensonal (3D) optical cryoimaging of the perfused heart before, during, and after IR.
Methods: The study involved four groups of hearts: time control (TC: heart perfusion without IR), TC followed by global ischemia (Isch), ischemia followed by reperfusion (IR), and TC with PCP (a mitochondrial uncoupler) perfusion. Mitochondrial NADH and FAD autofluorescence signals were recorded spectrofluorometrically online in guinea pig ex vivo-perfused hearts in the Langendorff mode. At the end of each specified protocol, hearts were rapidly removed and snap frozen in liquid N2 for later 3D optical cryoimaging of the mitochondrial NADH, FAD and NADH/FAD redox ratio (RR).
Results: The TC hearts revealed a heterogeneous spatial distribution of NADH, FAD and RR. Ischemia and IR altered the spatial distribution and caused an overall increase and decrease in the RR by 55% and 64%, respectively. Uncoupling with PCP resulted in the lowest level of the RR (73% oxidation) compared to TC.
Conclusion: The 3D optical cryoimaging of the heart provides novel insights into the heterogeneous distribution of mitochondrial NADH, FAD, RR, and metabolism from the base to the apex during ischemia and IR. This 3D information of the mitochondrial redox state in the normal and ischemic heart was not apparent in the dynamic spectrofluorometric data.
Editor’s Comment
Clinical Editor, Steven Schachter, MD

Heart disease is the leading cause of death for both men and women in the United States. Coronary heart disease is the most common type of heart disease and a major underlying reason for cardiac ischemic and reperfusion injury, which can lead to myocardial death. The cellular basis for myocardial injury in the setting of ischemia is heavily dependent on the redox state of myocardial mitochondria and therefore a better understanding of the mechanisms by which mitochondrial production of ATP is impaired due to ischemia could potentially have implications for the development of cardioprotective strategies in at-risk patients.
Ranji et al. used optical cryoimaging of mitochondrial NADH, FAD and NADH/FAD redox ratio to investigate the topographic consistency of the myocardial mitochondrial redux state in guinea pig hearts during ischemia and reperfusion. Compared to normal hearts, they showed significant redox heterogeneity across the myocardium that was not evident from dynamic spectrofluorometric assessment. As the authors suggest, this innovative work has implications with regard to use of optical flurorescence techniques to better delineate at-risk myocardial tissue in patients with heart disease and thereby provide an anatomic target for mitochondria-specific therapy.