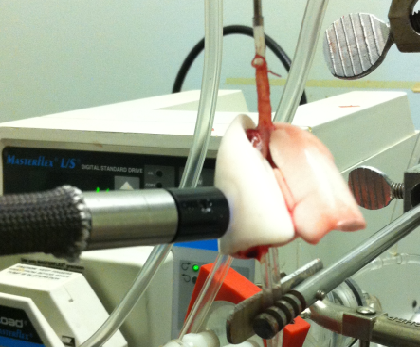
Recently we demonstrated the utility of optical fluorometry to detect a change in the redox status of mitochondrial autofluorescent coenzymes NADH (Nicotinamide Adenine Dinucleotide) and FAD (oxidized form of Flavin Adenine Dinucleotide (FADH2,)) as a measure of mitochondrial function in isolated perfused rat lungs (IPL). The objective of this study was to utilize optical fluorometry to evaluate the effect of rat exposure to hyperoxia (>95% O2 for 48 hours) on lung tissue mitochondrial redox status of NADH and FAD in a nondestructive manner in IPL. Surface NADH and FAD signals were measured before and after lung perfusion with perfusate containing rotenone (ROT, complex I inhibitor), potassium cyanide (KCN, complex IV inhibitor), and/or pentachlorophenol (PCP, uncoupler). ROT- or KCN-induced increase in NADH signal is considered a measure of complex I activity, and KCN-induced decrease in FAD signal is considered a measure of complex II activity. The results show that hyperoxia decreased complex I and II activities by 63% and 55%, respectively, as compared to lungs of rats exposed to room air (normoxic rats). Mitochondrial complex I and II activities in lung homogenates were also lower (77% and 63%, respectively) for hyperoxic than for normoxic lungs. These results suggest that the mitochondrial matrix is more reduced in hyperoxic lungs than in normoxic lungs, and demonstrate the ability of optical fluorometry to detect a change in mitochondrial redox state of hyperoxic lungs prior to histological changes characteristic of hyperoxia.
View full article