This Article is Featured in the Special Issue NIH-IEEE POCT 2015
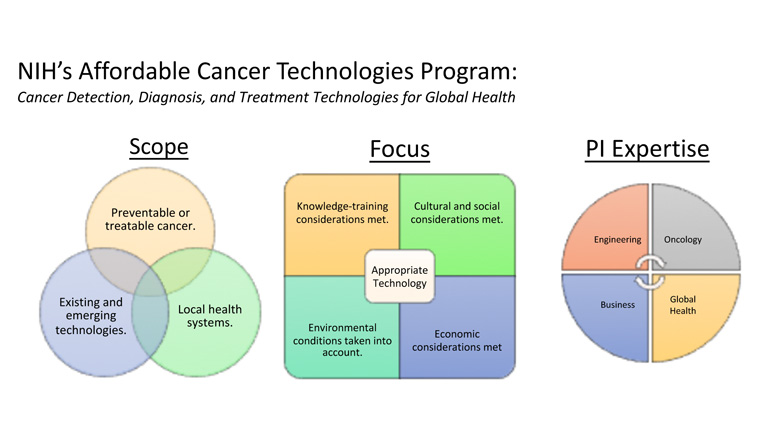
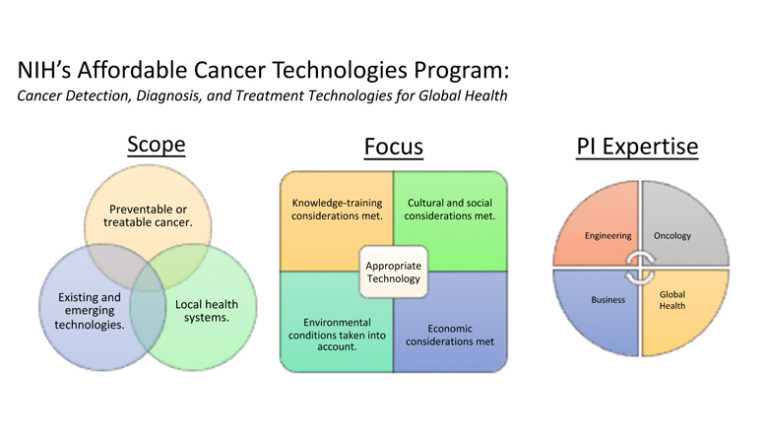
Point-of-care (POC) technologies have proved valuable in cancer detection, diagnosis, monitoring, and treatment in the developed world, and have shown promise in low-and-middle-income countries (LMIC) as well. Despite this promise, the unique design constraints presented in low-resource settings, coupled with the variety of country-specific regulatory and institutional dynamics, have made it difficult for investigators to translate successful POC cancer interventions to the LMIC markets. In response to this need, the National Cancer Institute has partnered with the National Institute of Biomedical Imaging and Bioengineering to create the National Institutes of Health Affordable Cancer Technologies (ACTs) program. This program seeks to simplify the pathway to market by funding multidisciplinary investigative teams to adapt and validate the existing technologies for cancer detection, diagnosis, and treatment in LMIC settings. The various projects under ACTs range from microfluidic cancer diagnostic tools to novel treatment devices, each geared for successful clinical adaptation to LMIC settings. Via progression through this program, each POC innovation will be uniquely leveraged for successful clinical translation to LMICs in a way not before seen in this arena.