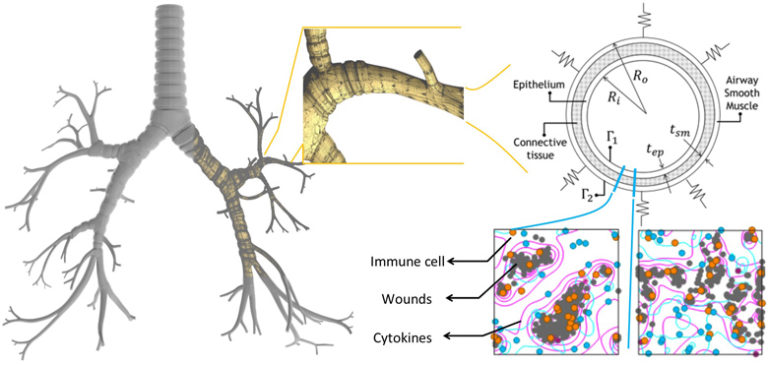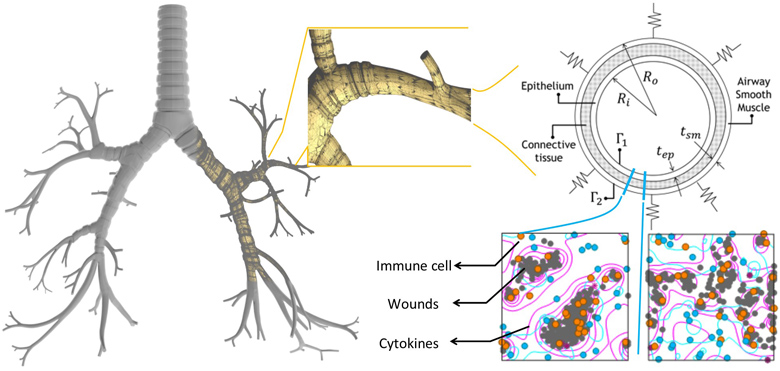
Ventilation-induced lung injury is a common problem faced by patients with respiratory problems who require mechanical ventilation (MV). This injury may lead to a greater chance of developing or exacerbating the acute respiratory distress syndrome which further complicates the therapeutic use of MV. The chain of events begins with the MV initiating an immune response that leads to inflammation induced tissue material alteration (stiffening) and eventually the loss of lung resistance. It is clear from this sequence of events that the phenomenon of ventilation induced injury is multi-scale by nature and, hence, requires holistic analysis involving simulations and informatics. An effective approach to this problem is to break it down into several major physical models. Each physical model is developed separately and can be seen as a component in a larger system that comprises the scale of the problem being investigated. In this paper, a multi-scale system consisting of breathing mechanics, tissue deformation, and cellular mechanics models is developed to assess the immune response. To demonstrate the potential of the model, a fluid–solid model is employed for breathing mechanics, a plane-strain elasticity model is applied to assess tissue deformation, and a cellular automata (CA) model is developed to account for immune response. A case study of three lower airways is presented. The CA model shows that this increased the immune response by five times, which correlates with alteration in the tissue microstructure. This alteration in turn is reflected in the material constant value obtained in the tissue mechanics model. However, the changes in strain rates in the airways after inflammation (and hence, lung compliance) were not as significant as the rates of change in immune response. Finally, results from the fluid–solid model demonstrate its potential for airflow characterization caused by tissue deformation that could lead to disease identification.