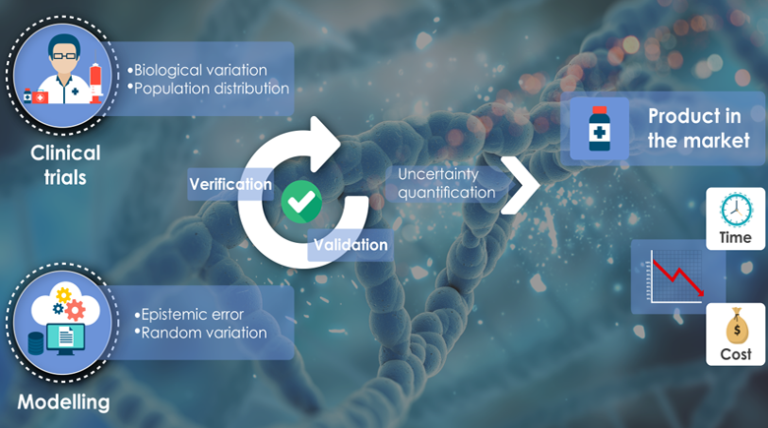
 Different research communities have developed various approaches to assess the credibility of predictive models. Each approach usually works well for a specific type of model, and under some epistemic conditions that are normally satisfied within that specific research domain. Recently, some regulatory agencies are starting to consider evidences of safety and efficacy on new medical products obtained using computer modelling and simulation (which is referred to as In Silico Trials); this has raised the attention in the computational medicine research community on the regulatory science aspects of this emerging discipline but, at the same time, has posed a foundational problem: the use of computer modelling is relatively recent in the domain of biomedical research, without a widely accepted epistemic framing for model credibility. Also, because of the inherent complexity of living organisms, biomedical modelers tend to use a variety of modelling methods, sometimes mixing them in the solution of a single problem. In such a context merely adopting credibility approaches developed within other research communities might not be appropriate. We recapitulate the most important concepts and practices for credibility assessment, including aspects regarding verification, validation and uncertainty quantification of computational models. Furthermore, we propose a theoretical framing for assessing the credibility of predictive models for In Silico Trials, which accounts for the epistemic specificity of this research field and is general enough to be used for different type of models. We also propose the mathematical formalisms for one particular application of In Silico methods, where validated predictive models are employed to augment clinical trials on physical cohorts through the simulation of the clinical trial of a new intervention on virtual cohorts. The potential scope for in silico methods is of course much broader: in principle in silico methods can be used also for reducing, refining, or replacing experimentations done in vitro, or in vivo.
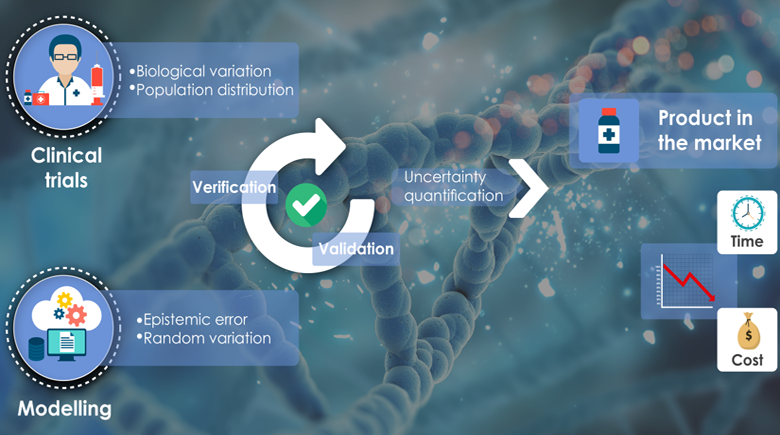
Different research communities have developed various approaches to assess the credibility of predictive models. Each approach usually works well for a specific type of model, and under some epistemic conditions that are normally satisfied within that specific research domain. Recently, some regulatory agencies are starting to consider evidences of safety and efficacy on new medical products obtained using computer modelling and simulation (which is referred to as In Silico Trials); this has raised the attention in the computational medicine research community on the regulatory science aspects of this emerging discipline but, at the same time, has posed a foundational problem: the use of computer modelling is relatively recent in the domain of biomedical research, without a widely accepted epistemic framing for model credibility. Also, because of the inherent complexity of living organisms, biomedical modelers tend to use a variety of modelling methods, sometimes mixing them in the solution of a single problem. In such a context merely adopting credibility approaches developed within other research communities might not be appropriate. We recapitulate the most important concepts and practices for credibility assessment, including aspects regarding verification, validation and uncertainty quantification of computational models. Furthermore, we propose a theoretical framing for assessing the credibility of predictive models for In Silico Trials, which accounts for the epistemic specificity of this research field and is general enough to be used for different type of models. We also propose the mathematical formalisms for one particular application of In Silico methods, where validated predictive models are employed to augment clinical trials on physical cohorts through the simulation of the clinical trial of a new intervention on virtual cohorts. The potential scope for in silico methods is of course much broader: in principle in silico methods can be used also for reducing, refining, or replacing experimentations done in vitro, or in vivo.