Around 50 million people in the United States live with—and suffer from—chronic pain. While some pain patients receive relief from physical therapy, medication, or surgery, others aren’t helped by these treatments. “It’s a debilitating situation,” says Ryan Lakin, divisional vice president of R&D at Abbott. “Patients have trouble just living a normal life, doing a lot of things that we take for granted.”
The next line of treatment often involves implantable neurostimulation devices that deliver electrical pulses to the spinal cord or peripheral nerves, interrupting pain signals before they reach the brain. While doctors have used neurostimulation for pain since the 1960s, neurostimulation devices and protocols continue to evolve in the quest to offer robust pain relief without side effects.
Reimagining spinal cord stimulation
One of the most common neurostimulation techniques for chronic pain is spinal cord stimulation (SCS), in which lead wires with electrodes are placed in the epidural space between the vertebrae and the spinal cord. Connected to these leads is a small pulse generator placed under the skin, similar to a pacemaker. A handheld controller can be used to change stimulation parameters.
New technological advances have improved the efficacy of SCS devices and the user experience. One recent example is Abbott’s Proclaim XR, which received approval from the U.S. Food and Drug Administration (FDA) last year (Figure 1). While many SCS systems require daily recharging, Proclaim XR is a recharge-free device that can last as long as ten years. Proclaim XR delivers a novel, proprietary stimulation waveform called BurstDR. The BurstDR waveform delivers intermittent bursts of stimulation, requiring less energy than traditional tonic stimulation. “Alternate stimulation systems typically use higher frequencies, higher amplitude signals as well as more constant therapy,” says Lakin. “When they do that, that typically requires more energy.”
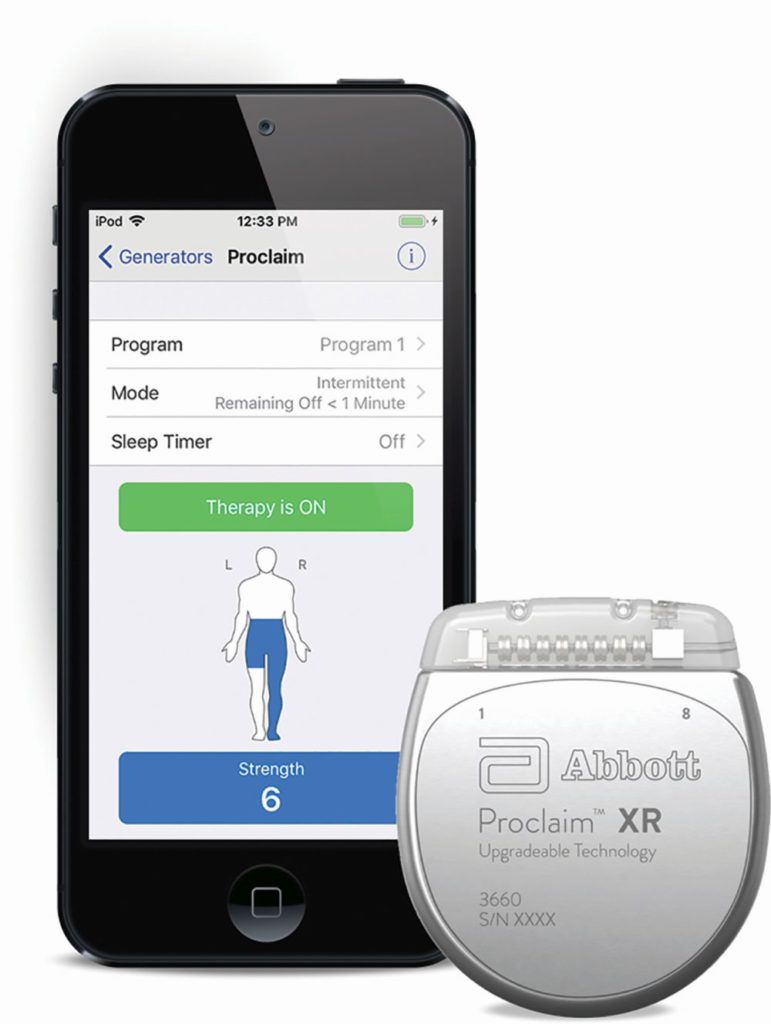
According to Lakin, BurstDR mimics natural firing patterns found in the brain. A nonlinear charge accumulation phase in the waveform creates a signal that activates both the medial and lateral pain pathways in the brain, disrupting both the physical and emotional aspects of pain. A randomized control trial of 100 patients found that patients experienced better pain relief with BurstDR stimulation than with tonic stimulation and 70.8% preferred the BurstDR stimulation to tonic stimulation at the end of the 12 week trial [1].
BurstDR stimulation is also “dosable,” meaning the on:off stimulation protocol is customizable. In one study, 46% of patients chose to continue using the lowest dose protocol (30 seconds stim, 360 seconds off), and 71% used stimulation for less than 4 hours a day [2]. An imaging study in rats presented at the 17th Annual Pain Medicine Meeting suggests that this intermittent dosing works because burst stimulation creates a “carry over” effect, where pain relief continues for a period after the stimulation is turned off [3].
Newer SCS systems from Abbott and other companies have also been able to offer patients pain relief without eliciting paresthesia, a sort of tingling sensation that is used to cover up pain in some SCS protocols. Abbott’s SCS systems are upgradeable so that as new protocols are optimized, people with existing devices can upgrade their stimulation parameters. Patients also have the option to control their stimulator from a smartphone.
In addition, a new randomized control trial is testing whether Proclaim XR can be used to successfully help people with low back pain who are not optimal surgery candidates. Currently, the device is not labeled for that indication, but “if approved it could help a large number of patients,” says Lakin.
The effects of SCS can be profound for chronic pain patients, many of whom have struggled with daily pain and disability. Lakin recounts having dinner with a young veteran who had been in debilitating pain for ten years. “He went to a family reunion where he hadn’t seen some people for a few years, and people didn’t even recognize him after he got a spinal cord stimulator,” says Lakin. “He was walking tall and proud. He no longer had a cane. He was happy. And he was really just so thrilled that he was able to get his life back.”
Rethinking peripheral nerve stimulation
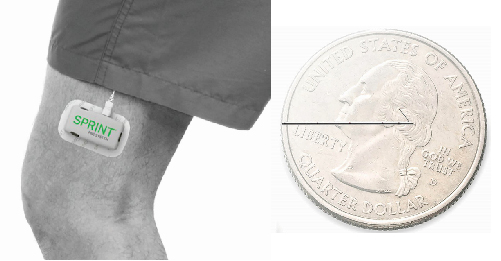
Another technique for treating chronic pain is peripheral nerve stimulation (PNS), which targets the nerves that connect regions of the body to the spinal cord [4]. Unlike other peripheral nerve stimulators, SPRINT PNS from SPR Therapeutics is specifically designed for short-term use—a maximum of 60 days—and has a battery pack that is attached to the skin [Figure 2(a)].
SPRINT PNS works by targeting the peripheral nerves specific to the pain site and essentially “resetting” the signals these nerves send to the brain. “People who have chronic pain, they’re essentially in a sensitized state,” says Josh Boggs, Ph.D., senior vice president of research and development at SPR Therapeutics. “Our goal with this system is to provide a break from the pain and send in healthy and comfortable signals that are designed to help rebalance the input that the brain is receiving from the periphery and unwind that central pain state.”
To deliver electrical stimulation, the SPRINT PNS lead is placed near the peripheral nerve but not too close—something Boggs says is a key difference from other peripheral nerve stimulators [Figure 2(b)]. “Before we developed our system the theme of PNS was always, location, location, location—just like real estate—get the leads as close as you can to the nerve,” says Boggs. “But the challenge with that is that as you try to increase the intensity to reach the pain-relieving fibers on the other side of the nerve, you can accidentally activate the pain fibers.”
Compared to some other PNS devices, the longer, larger SPRINT PNS electrode provides a stronger signal further away from the nerve that activates more of the fibers throughout the nerve that send nonpain sensory signals to the brain. A typical stimulation protocol for activating these sensory fibers is 100 Hz for 24 hours. A different stimulation protocol—12 Hz for 6–12 hours daily—can be used to activate motor fibers, which can help people with low back pain.
Because the SPRINT PNS electrode is placed further away from the nerve, it is more forgiving for the physician placing the lead. “We’ve sort of widened the bullseye, if you will, to make it easier to hit,” says Boggs. “We wanted to develop a system that didn’t require neurosurgical expertise to place the leads,” says Boggs.
Boggs says this placement is also more forgiving for the patient. With pain relief, patients start becoming more active and this activity can cause an electrode to move. Since the SPRINT PNS electrode is further from the nerve and has a larger stimulating radius, this may be less of an issue than for other devices. The SPRINT PNS electrode is also a different shape from traditional tube-like electrodes—it is coiled like a spring or a telephone cord—and more flexible. “As the patient starts moving, it’s going to put tension and forces on the lead and so we wanted to use a lead that’s really designed for that type of movement,” says Boggs. The coil structure also promotes tissue regrowth around the electrode, helping seal off the site where the leads exit the skin and preventing infection. This tissue growth also prevents “pistoning” of the electrode and helps secure it in place.
SPRINT PNS has been tested on one of the trickiest pain conditions to treat: postamputation pain. A double-blind randomized control study found 58% of patients receiving SPRINT PNS stimulation experienced 50% or greater reduction in postamputation pain compared to 14% in the placebo group, which had no stimulation from their device [5]. SPRINT PNS is also used to treat other conditions, including chronic pain in the back, arms, legs, feet, and shoulders, postsurgical pain, neuropathic pain, and complex regional pain syndrome.
SPRINT PNS doesn’t work for every patient—some patients don’t experience pain relief from the stimulation and others have their pain return after the device is removed—but many patients do experience an improvement within 60 days. In general, “about 75% of the patients have a significant and positive response and have sustained relief,” says Boggs.
Restorative neurostimulation
Mainstay Medical’s ReActiv8 is a new implantable neurostimulation system designed specifically to treat chronic low back pain (Figure 3). Chronic low back pain is defined as low back pain that persists for more than 90 days. “It’s a brutal condition,” says Dr. Chris Gilligan, MD, MBA, chief of the division of pain medicine at Brigham & Women’s Hospital and principal investigator of a ReActiv8 clinical trial. “When it’s that severe, it robs people, it takes people’s life away almost. They can’t do the things they need to do, they can’t do the things they want to do.”

Chronic back pain begins with a strained or sprained joint in the spine. The initial injury makes movement painful and so—to limit the pain—the brain suppresses the activation of back muscles. When these muscles are inactivated, however, they become weaker, making it easier for the spine to move into painful positions. This causes a continuous cycle of pain and muscle weakening.
ReActiv8 is an implanted neurostimulation device that breaks the pain-muscle weakening cycle by stimulating the dorsal ramus nerve (SPRINT PNS can also be used to stimulate the dorsal ramus nerve) [6]. Stimulating this nerve activates the lumbar multifidus muscle, an important stabilizing muscle in the low back, and restores neuromuscular control and spine stability.
“This is not covering up any sensation,” says Gilligan. “This [device] is working by restoring the function of the multifidus. You are restoring stability and if you restore stability, then the pain is going away,” says Gilligan. Because the goal is to restore muscle and spine stability, stimulation isn’t on all day, instead the patient turns on the device twice a day for 30 minutes. “It’s really a completely new approach,” says Gilligan. “It’s restorative neurostimulation as opposed to what we’ve done for years and years and years, which are different technologies for palliative stimulation, covering up the pain.”
According to Gilligan, this new mechanism of action provides hope to chronic back pain patients who have exhausted other options including physical therapy, medication, and steroid injection and who are not good candidates for spinal surgery. “Previously, somebody in that circumstance for the most part was out of luck,” he says. A study of 53 patients who had experienced an average of 14 years of back pain found that 58% of patients responded to the ReActiv8 implant, meaning that they showed at least two points of improvement on a 1–10 pain scale at 90 days [7]. At the end of a year with the device, the average pain score dropped from 6.8 to 2.4. Average disability and quality of life scores also improved significantly.
An international randomized active control clinical study of 204 patients found that, after 120 days, there was not a significant difference in the percentage of patients who responded to the normal ReActiv8 stimulation protocol versus the group who received low level control stimulation [8]. However, after one year of receiving active stimulation, 60% of patients had greater than 50% pain reduction (the control group was switched to active simulation after 120 days). Additionally, of the 50 patients who were taking opioids at the beginning of the study, 44% had eliminated or significantly reduced their use at the one year mark. “Patients continue to improve over time,” says Gilligan. “We see significant improvements, for example, at four months of therapy, but patients are doing much better in one year than they were at four months.”
The FDA approved ReActiv8 for the treatment of chronic low back pain in June 2020. Mainstay Medical plans to commercially launch the device in the United States in early 2021. The device is already in routine clinical practice in parts of Europe.
According to Gilligan, the improvements seen in many of the patients who have tried ReActiv8 have been life-changing. “There was a young woman in the United Kingdom who was a mounted police officer and couldn’t work anymore because of her back pain,” says Gilligan. He says with her use of ReActiv8 she was able to get back work—and back on her horse—and even ran the London Marathon. “I’m very excited to have something where we can try to help some of these patients, and it’s something that appears overall very safe,” he says.
References
- T. Deer et al., “Success using neuromodulation with BURST (SUNBURST) study: Results from a prospective, randomized controlled trial using a novel burst waveform,” Neuromodulation, vol. 21, no. 1, pp. 56–66, 2017.
- T. R. Deer et al., “Novel intermittent dosing burst paradigm in spinal cord stimulation: Intermittent dosing burst SCS,” Neuromodulation, no. ner.13143, 2020, Epub ahead of print.
- M. Saber et al., “Rat fMRI brain responses to noxious stimulation during tonic, burst, and burst-microdosing spinal cord stimulation,” in Proc. 17th Ann. Pain Medicine Meeting, 2019.
- R. D. Wilson and C. H. Kim, “Percutaneous and implanted peripheral nerve stimulation for the management of pain: Current evidence and future directions,” Curr. Phys. Med. Rehabil. Rep., vol. 8, no. 1, pp. 1–7, 2020.
- C. Gilmore et al., “Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial,” Reg. Anesth. Pain Med., vol. 44, no. 6, pp. 637–645, 2019.
- C. A. Gilmore, J. Patel, L.-G. Esebua, and M. Burchell, “A review of peripheral nerve stimulation techniques targeting the medial branches of the lumbar dorsal rami in the treatment of chronic low back pain,” Pain Med., vol. 21, no. Suppl. 1, pp. S41–S46, 2020.
- K. Deckers et al., “New therapy for refractory chronic mechanical low back pain-restorative neurostimulation to activate the lumbar multifidus: One year results of a prospective multicenter clinical trial: Restorative neurostimulation for clbp,” Neuromodulation, vol. 21, no. 1, pp. 48–55, 2018.
- “ReActiv8 implantable neurostimulation system for chronic low back pain,” Clinicaltrials.gov. Accessed: Dec. 9, 2020. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT02577354



